THE
CAMBRIDGE NATURAL HISTORY
S. F. HARMER, Sc.D., F.R.S., Fellow of King’s College, Cambridge; Keeper of the Department of Zoology in the British Museum (Natural History)
A. E. SHIPLEY, M.A., Fellow and Tutor of Christ’s College, Cambridge; Reader in Zoology in the University
CRUSTACEA
By Geoffrey Smith, M.A. (Oxon.), Fellow of New College, Oxford; and the late W. F. R. Weldon, M.A. (D.Sc., Oxon.), formerly Fellow of St. John’s College, Cambridge, and Linacre Professor of Human and Comparative Anatomy, Oxford
TRILOBITES
By Henry Woods, M.A., St. John’s College, Cambridge; University Lecturer in Palaeozoology
INTRODUCTION TO ARACHNIDA, AND KING-CRABS
By A. E. Shipley, M.A., F.R.S., Fellow and Tutor of Christ’s College, Cambridge; Reader in Zoology
EURYPTERIDA
By Henry Woods, M.A., St. John’s College, Cambridge; University Lecturer in Palaeozoology
SCORPIONS, SPIDERS, MITES, TICKS, ETC.
By Cecil Warburton, M.A., Christ’s College, Cambridge; Zoologist to the Royal Agricultural Society
TARDIGRADA (WATER-BEARS)
By A. E. Shipley, M.A., F.R.S., Fellow and Tutor of Christ’s College, Cambridge; Reader in Zoology
PENTASTOMIDA
By A. E. Shipley, M.A., F.R.S., Fellow and Tutor of Christ’s College, Cambridge; Reader in Zoology
PYCNOGONIDA
By D’Arcy W. Thompson, C.B., M.A., Trinity College, Cambridge; Professor of Natural History in University College, Dundee
All the ingenious men, and all the scientific men, and all the fanciful men, in the world, with all the old German bogypainters into the bargain, could never invent ... anything so curious, and so ridiculous, as a lobster.
PREFACE
The Editors feel that they owe an apology and some explanation to the readers of The Cambridge Natural History for the delay which has occurred in the issue of this, the fourth in proper order, but the last to appear of the ten volumes which compose the work. The delay has been due principally to the untimely death of Professor W. F. E. Weldon, who had undertaken to write the Section on the Crustacea. The Chapter on the Branchiopoda is all he actually left ready for publication, but it gives an indication of the thorough way in which he had intended to treat his subject. He had, however, superintended the preparation of a number of beautiful illustrations, which show that he had determined to use, in the main, first-hand knowledge. Many of these figures have been incorporated in the article by Mr. Geoffrey Smith, to whom the Editors wish to express their thanks for taking up, almost at a moment’s notice, the task which had dropped from his teacher’s hand.
A further apology is due to the other contributors to this volume. Their contributions have been in type for many years, and owing to the inevitable delays indicated above they have been called upon to make old articles new, ever an ungrateful labour.
The appearance of this volume completes the work the Editors embarked on some sixteen years ago. It coincides with the cessation of an almost daily intercourse since the time when they “came up” to Cambridge as freshmen in 1880.
March 1909.
CONTENTS
| PAGE | |
|---|---|
| Scheme of the Classification adopted in this Volume | xi |
| CRUSTACEA | |
| CHAPTER I | |
| CRUSTACEA | |
| General Organisation | 3 |
| CHAPTER II | |
| CRUSTACEA (continued) | |
| Entomostraca—Branchiopoda—Phyllopoda—Cladocera—Water-fleas | 18 |
| CHAPTER III | |
| CRUSTACEA ENTOMOSTRACA (continued) | |
| Copepoda | 55 |
| CHAPTER IV | |
| CRUSTACEA ENTOMOSTRACA (continued) | |
| Cirripedia—Phenomena of Growth and Sex—Ostracoda | 79 |
| viii | |
| CHAPTER V | |
| CRUSTACEA (continued) | |
| Malacostraca: Leptostraca—Phyllocarida: Eumalacostraca: Syncarida—Anaspidacea: Peracarida—Mysidacea—Cumacea—Isopoda—Amphipoda: Hoplocarida—Stomatopoda | 110 |
| CHAPTER VI | |
| CRUSTACEA MALACOSTRACA (continued) | |
| Eumalacostraca (CONTINUED): Eucarida—Euphausiacea—Compound Eyes—Decapoda | 144 |
| CHAPTER VII | |
| CRUSTACEA (continued) | |
| Remarks on the Distribution of Marine and Fresh-water Crustacea | 197 |
| CHAPTER VIII | |
| CRUSTACEA (continued) | |
| Trilobita | 221 |
| ARACHNIDA | |
| CHAPTER IX | |
| Arachnida—Introduction | 255 |
| CHAPTER X | |
| ARACHNIDA (continued) | |
| Delobranchiata = Merostomata—Xiphosura | 259 |
| CHAPTER XI | |
| ARACHNIDA DELOBRANCHIATA (continued) | |
| Eurypterida = Gigantostraca | 283 |
| ix | |
| CHAPTER XII | |
| ARACHNIDA (continued) | |
| Embolobranchiata—Scorpionidea—Pedipalpi | 297 |
| CHAPTER XIII | |
| ARACHNIDA EMBOLOBRANCHIATA (continued) | |
| Araneae—External Structure—Internal Structure | 314 |
| CHAPTER XIV | |
| ARACHNIDA EMBOLOBRANCHIATA (continued) | |
| Araneae (CONTINUED)—Habits—Ecdysis—Treatment of Young—Migration—Webs—Nests—Egg-cocoons—Poison—Fertility—Enemies—Protective Coloration—Mimicry—Senses—Intelligence—Mating Habits—Fossil Spiders | 338 |
| CHAPTER XV | |
| ARACHNIDA EMBOLOBRANCHIATA (continued) | |
| Araneae (CONTINUED)—Classification | 384 |
| CHAPTER XVI | |
| ARACHNIDA EMBOLOBRANCHIATA (continued) | |
| Palpigradi—Solifugae = Solpugae—Chernetidea = Pseudoscorpiones | 422 |
| CHAPTER XVII | |
| ARACHNIDA EMBOLOBRANCHIATA (continued) | |
| Podogona = Ricinulei—Phalangidea = Opiliones—Habits—Structure—Classification | 439 |
| CHAPTER XVIII | |
| ARACHNIDA EMBOLOBRANCHIATA (continued) | |
| Acarina—Harvest-Bugs—Parasitic Mites—Ticks—Spinning Mites—Structure—Metamorphosis—Classification | 454 |
| x | |
| CHAPTER XIX | |
| ARACHNIDA (APPENDIX I) | |
| Tardigrada—Occurrence—Ecdysis—Structure—Development—Affinities—Biology—Desiccation—Parasites—Systematic | 477 |
| CHAPTER XX | |
| ARACHNIDA (APPENDIX II) | |
| Pentastomida—Occurrence—Economic Importance—Structure—Development and Life-History—Systematic | 488 |
| PYCNOGONIDA | |
| CHAPTER XXI | |
| Pycnogonida | 501 |
| INDEX | 543 |
SCHEME OF THE CLASSIFICATION ADOPTED IN THIS VOLUME
| CRUSTACEA (p. 3). | |||||
| ENTOMOSTRACA (p. 18). | |||||
| Divisions. | Orders. | Sub-Orders. | Tribes. | Families. | |
|---|---|---|---|---|---|
| Branchiopoda (p. 18) | Phyllopoda (pp. 19, 35) | Branchipodidae (pp. 19, 35). | |||
| Apodidae (pp. 19, 36). | |||||
| Limnadiidae (pp. 20, 36). | |||||
| Cladocera (p. 37) | Calyptomera (pp. 38, 51) | Ctenopoda (p. 51) | Sididae (p. 51). | ||
| Holopediidae (p. 51). | |||||
| Anomopoda (p. 51) | Daphniidae (p. 51). | ||||
| Bosminidae (p. 53). | |||||
| Lyncodaphniidae (p. 53). | |||||
| Lynceidae = Chydoridae (p. 53). | |||||
| Gymnomera (pp. 38, 54) | Polyphemidae (p. 54). | ||||
| Leptodoridae (p. 54). | |||||
| Divisions. | Orders. | Sub-Orders. | Tribes. | Families. | |
| Copepoda (p. 55) | Eucopepoda (p. 57) | Gymnoplea (p. 57) | Amphascandria (p. 57) | Calanidae (p. 57). | |
| Heterarthrandria (p. 58) | Centropagidae (p. 58). | ||||
| Candacidae (p. 60). | |||||
| Pontellidae (p. 60). | |||||
| Podoplea (p. 61) | Ampharthrandria (p. 61) | Cyclopidae (pp. 61, 62). | |||
| Harpacticidae (pp. 61, 62). | |||||
| Peltiidae (p. 63). | |||||
| Monstrillidae (p. 63). | |||||
| Ascidicolidae (p. 66). | |||||
| Asterocheridae (p. 67). | |||||
| Dichelestiidae (p. 68). | |||||
| xii | Isokerandria (p. 69) | Oncaeidae (p. 69). | |||
| Corycaeidae (p. 69). | |||||
| Lichomolgidae (p. 70). | |||||
| Ergasilidae (p. 71). | |||||
| Bomolochidae (p. 71). | |||||
| Chondracanthidae (p. 72). | |||||
| Philichthyidae (p. 73). | |||||
| Nereicolidae (p. 73). | |||||
| Hersiliidae (p. 73). | |||||
| Caligidae (p. 73). | |||||
| Lernaeidae (p. 74). | |||||
| Lernaeopodidae (p. 75). | |||||
| Choniostomatidae (p. 76). | |||||
| Branchiura (P. 76) | Argulidae (p. 76). | ||||
| Cirripedia (p. 79) | Pedunculata (p. 84) | Polyaspidae (p. 84). | |||
| Pentaspidae (p. 87). | |||||
| Tetraspidae (p. 88). | |||||
| Anaspidae (p. 89). | |||||
| Operculata (p. 89) | Verrucidae (p. 91). | ||||
| Octomeridae (p. 91). | |||||
| Hexameridae (p. 91). | |||||
| Tetrameridae (p. 92). | |||||
| Acrothoracica (p. 92). | |||||
| Ascothoracica (p. 93). | |||||
| Apoda (p. 94). | |||||
| Rhizocephala (p. 95). | |||||
| Ostracoda (p. 107) | Cypridae (p. 107). | ||||
| Cytheridae (p. 107). | |||||
| Halocypridae (p. 108). | |||||
| Cypridinidae (p. 108). | |||||
| Polycopidae (p. 109). | |||||
| Cytherellidae (p. 109). | |||||
| MALACOSTRACA (p. 110). | |||||
| LEPTOSTRACA (p. 111) | |||||
| Phyllocarida (p. 111). | |||||
| xiii | |||||
| Divisions. | Orders. | Sub-Orders. | Tribes | Families. | |
| EUMALACOSTRACA (p. 112). | |||||
| Syncarida (p. 114) | Anaspidacea (p. 115) | Anaspididae (p. 115). | |||
| Koonungidae (p. 117). | |||||
| Peracarida (p. 118) | Mysidacea (p. 118) | Eucopiidae (p. 118). | |||
| Lophogastridae (p. 119). | |||||
| Mysidae (p. 119). | |||||
| Cumacea (p. 120) | Cumidae (p. 121). | ||||
| Lampropidae (p. 121). | |||||
| Leuconidae (p. 121). | |||||
| Diastylidae (p. 121). | |||||
| Pseudocumidae (p. 121). | |||||
| Isopoda (p. 121) | Chelifera (p. 122) | Apseudidae (p. 122). | |||
| Tanaidae (p. 122). | |||||
| Flabellifera (p. 124) | Anthuridae (p. 124). | ||||
| Gnathiidae (p. 124). | |||||
| Cymothoidae (p. 126). | |||||
| Cirolanidae (p. 126). | |||||
| Serolidae (p. 126). | |||||
| Sphaeromidae (p. 126). | |||||
| Valvifera (p. 127) | Idotheidae (p. 127). | ||||
| Arcturidae (p. 127). | |||||
| Asellota (p. 127) | Asellidae (p. 128). | ||||
| Munnopsidae (p. 128). | |||||
| Oniscoida (p. 128). | |||||
| Epicarida (p. 129) | Cryptoniscina (pp. 129, 130) | Microniscidae (p. 130). | |||
| Cryptoniscidae (p. 130). | |||||
| Liriopsidae (p. 130). | |||||
| Hemioniscidae (p. 130). | |||||
| Cabiropsidae (p. 130). | |||||
| Podasconidae (p. 130). | |||||
| Asconiscidae (p. 130). | |||||
| Bopyrina (pp. 129, 130, 132) | Dajidae (p. 130). | ||||
| Phryxidae (p. 130). | |||||
| Bopyridae (pp. 130, 133). | |||||
| Entoniscidae (pp. 130, 134). | |||||
| Phreatoicidea (p. 136) | Phreatoicidae (p. 136) | ||||
| Amphipoda (p. 136) | Crevettina (p. 137) | Lysianassidae (p. 137). | |||
| Haustoriidae (p. 137). | |||||
| Gammaridae (p. 138). | |||||
| Talitridae (p. 139). | |||||
| Corophiidae (p. 139). | |||||
| Laemodipoda (p. 139) | Caprellidae (p. 139). | ||||
| Cyamidae (p. 140). | |||||
| Hyperina (p. 140). | |||||
| Hoplocarida (p. 141) | Stomatopoda (p. 141) | Squillidae (p. 143). | |||
| Eucarida (p. 144) | Euphausiacea (p. 144) | Euphausiidae (p. 144). | |||
| Decapoda (p. 152) | Macrura (p. 153) | Nephropsidea (p. 154) | Nephropsidae (p. 154). | ||
| Astacidae (p. 157). | |||||
| Parastacidae (p. 157). | |||||
| Eryonidea (p. 157) | Eryonidae (p. 158). | ||||
| Peneidea (pp. 158, 162) | Peneidae (p. 162). | ||||
| Sergestidae (p. 162). | |||||
| Stenopodidae (p. 162). | |||||
| Caridea (pp. 158, 163) | Pasiphaeidae (p. 163). | ||||
| Acanthephyridae (p. 163). | |||||
| Atyidae (p. 163). | |||||
| Alpheidae (p. 163). | |||||
| Psalidopodidae (p.164). | |||||
| Pandalidae (p. 164). | |||||
| Hippolytidae (p. 164). | |||||
| Palaemonidae (p. 164). | |||||
| Glyphocrangonidae (p. 164). | |||||
| Crangonidae (p. 164). | |||||
| Loricata (p. 165) | Palinuridae (p. 167). | ||||
| Scyllaridae (p. 167). | |||||
| Thalassinidea (p. 167) | Callianassidae (p. 167). | ||||
| Anomura (p. 167) | Galatheidea (p. 168) | Aegleidae (p. 169). | |||
| Galatheidae (p. 169). | |||||
| Porcellanidae (p. 170). | |||||
| Hippidea (p. 170) | Albuneidae (p. 171). | ||||
| Hippidae (p. 171). | |||||
| Paguridea (p. 171) | Pylochelidae (p. 180). | ||||
| Paguridae (p. 180). | |||||
| Eupagurinae (p. 180). | |||||
| Pagurinae (p. 180). | |||||
| Coenobitidae (p. 181). | |||||
| Lithodidae (p. 181). | |||||
| Hapalogastorinae (p. 181). | |||||
| Lithodinae (p. 181). | |||||
| Brachyura (p. 181) | Dromiacea (p. 183) | Dromiidae (p. 184). | |||
| Dynomenidae (p. 184). | |||||
| Homolidae (p. 184). | |||||
| Oxystomata (p. 185) | Calappidae (p. 187). | ||||
| Leucosiidae (p. 188). | |||||
| Dorippidae (p. 188). | |||||
| Raninidae (p. 188). | |||||
| Cyclometopa (p. 188) | Corystidae (p. 190). | ||||
| Atelecyclidae (p. 190). | |||||
| Cancridae (p. 191). | |||||
| Portunidae (p. 191). | |||||
| Xanthidae (p. 191). | |||||
| Thelphusidae = Potamonidae (p. 191). | |||||
| Oxyrhyncha (p. 191) | Maiidae (p. 193). | ||||
| Parthenopidae (p. 193). | |||||
| Hymenosomatidae (p. 193). | |||||
| Catometopa (p. 193) | Carcinoplacidae (p. 195). | ||||
| Gonoplacidae (p. 195). | |||||
| Pinnotheridae (p. 195). | |||||
| Grapsidae (p. 196). | |||||
| Gecarcinidae (p. 196). | |||||
| Ocypodidae (p. 196). | |||||
| ARACHNIDA (p. 255). | |||
| DELOBRANCHIATA = MEROSTOMATA (pp. 258, 259). | |||
| Orders. | Sub-Orders. | Families. | Sub-Families. |
|---|---|---|---|
| Xiphosura (pp. 258, 259, 276) | Xiphosuridae (p. 276) | Xiphosurinae (p. 276). | |
| Tachypleinae (p. 276). | |||
| Eurypterida = Gigantostraca (pp. 258, 283) | Eurypteridae (p. 290). | ||
| EMBOLOBRANCHIATA (pp. 258, 297). | |||
| Scorpionidea (pp. 258, 297) | Buthidae (p. 306) | Buthinae (p. 306). | |
| Centrurinae (p. 306). | |||
| Scorpionidae (p. 306) | Diplocentrinae (p. 307). | ||
| Urodacinae (p. 307). | |||
| Scorpioninae (p. 307). | |||
| Hemiscorpioninae (p. 307). | |||
| Ischnurinae (p. 307). | |||
| Chaerilidae (p. 307). | |||
| Chactidae (p. 307) | Megacorminae (p. 308). | ||
| Euscorpiinae (p. 308). | |||
| Chactinae (p. 308). | |||
| Vejovidae (p. 308). | |||
| Bothriuridae (p. 308). | |||
| Pedipalpi (pp. 258, 308) | Thelyphonidae (p. 312). | ||
| Schizonotidae = Tartaridae (p. 312). | |||
| Tarantulidae = Phrynidae (p. 312) | Tarantulinae (p. 313). | ||
| Phrynichinae (p. 313). | |||
| Charontinae (p. 313). | |||
| Araneae (pp. 258, 314) | Liphistiidae (p. 386). | ||
| Aviculariidae = Mygalidae (p. 386) | Paratropidinae (p. 387). | ||
| Actinopodinae (p. 387). | |||
| Miginae (p. 387). | |||
| Ctenizinae (p. 388). | |||
| Barychelinae (p. 389). | |||
| Aviculariinae (p. 389). | |||
| Diplurinae (p. 390). | |||
| Atypidae (p. 390). | |||
| Filistatidae (p. 391). | |||
| Oecobiidae = Urocteidae (p. 392). | |||
| Sicariidae = Scytodidae (p. 393). | |||
| Hypochilidae (p. 393). | |||
| Leptonetidae (p. 393). | |||
| Oonopidae (p. 393). | |||
| Hadrotarsidae (p. 394). | |||
| Dysderidae (p. 394) | Dysderinae (p. 394). | ||
| Segestriinae (p. 395). | |||
| Caponiidae (p. 395). | |||
| Prodidomidae (p. 395). | |||
| Drassidae (p. 396) | Drassinae (p. 396). | ||
| Clubioninae (p. 397). | |||
| Liocraninae (p. 397). | |||
| Micariinae (p. 397). | |||
| Palpimanidae (p. 398). | |||
| Eresidae (p. 398). | |||
| Dictynidae (p. 398). | |||
| Psechridae (p. 399). | |||
| Zodariidae = Enyoidae (p. 399). | |||
| Hersiliidae (p. 400). | |||
| Pholcidae (p. 401). | |||
| Theridiidae (p. 401) | Argyrodinae (p. 402). | ||
| Episininae (p. 402). | |||
| Theridioninae (p. 403). | |||
| Phoroncidiinae (p. 404). | |||
| Erigoninae (p. 404). | |||
| Formicinae (p. 405). | |||
| Linyphiinae (p. 405). | |||
| Epeiridae (p. 406) | Theridiosomatinae (p. 407). | ||
| Tetragnathinae (p. 407). | |||
| Argiopinae (p. 408). | |||
| Nephilinae (p. 408). | |||
| Epeirinae (p. 408). | |||
| Gasteracanthinae (p. 409). | |||
| Poltyinae (p. 410). | |||
| Arcyinae (p. 410). | |||
| Uloboridae (p. 410) | Dinopinae (p. 410). | ||
| Uloborinae (p. 410). | |||
| Miagrammopinae (p. 411). | |||
| Archeidae (p. 411). | |||
| Mimetidae (p. 411). | |||
| Thomisidae (p. 412) | Thomisinae = Misumeninae (p. 412). | ||
| Philodrominae (p. 413). | |||
| Sparassinae (p. 414). | |||
| Aphantochilinae (p. 414). | |||
| Stephanopsinae (p. 414). | |||
| Selenopinae (p. 414). | |||
| Zoropsidae (p. 415). | |||
| Platoridae (p. 415). | |||
| Agelenidae (p. 415) | Cybaeinae (p. 415). | ||
| Ageleninae (p. 416). | |||
| Hahniinae (p. 416). | |||
| Nicodaminae (p. 416). | |||
| Pisauridae (p.416). | |||
| Lycosidae (p. 417). | |||
| Ctenidae (p. 418). | |||
| Senoculidae (p. 418). | |||
| Oxyopidae (p. 419). | |||
| Attidae = Salticidae (p. 419). | |||
| Palpigradi (pp. 258, 422). | |||
| Solifugae = Solpugae (pp. 258, 423) | Galeodidae (p. 428). | ||
| Solpugidae (p. 429) | Rhagodinae (p. 429). | ||
| Solpuginae (p. 429). | |||
| Daesiinae (p. 429). | |||
| Eremobatinae (p. 429). | |||
| Karshiinae (p. 429). | |||
| Hexisopodidae (p. 429). | |||
| Chernetidea = Chernetes = Pseudoscorpiones (pp. 258, 430) | Cheliferinae (p. 436). | Cheliferidae (p. 436) | |
| Garypinae (pp. 436, 437). | |||
| Obisiinae (pp. 436, 437). | |||
| Podogona = Ricinulei (pp. 258, 439) | Cryptosteinmatidae (p.440). | ||
| Phalangidea = Opiliones (pp. 258, 440) | Cyphophthalmi (p. 447) | Sironidae (p. 448). | |
| Mecostethi = Laniatores (p. 448) | Phalangodidae (p. 448). | ||
| Cosmetidae (p. 449). | |||
| Gonyleptidae (p. 449). | |||
| Plagiostethi = Palpatores (p. 449) | Phalangiidae (p. 449) | Sclerosomatinac (p.449). | |
| Phalangiinae (p. 450). | |||
| Ischyropsalidae (p. 451). | |||
| Nemastomatidae (p. 451). | |||
| Trogulidae (p. 452). | |||
| Acarina = Acari = Acaridea (pp. 258, 454) | Vermiformia (p. 464) | Eriophyidae = Phytoptidae (p. 464). | |
| Demodicidae (p. 465). | |||
| Astigmata (p. 465) | Sarcoptidae (p. 466) | Sarcoptinae (p. 466). | |
| Analgesinae (p. 466). | |||
| Tyroglyphinae (p. 466). | |||
| Metastigmata (p. 467) | Oribatidae (p. 467). | ||
| Ixodoidea (p. 468) | Argasidae (p. 469). | ||
| Ixodidae (p. 469). | |||
| Gamasidae (p. 470) | Gamasinae (p. 470). | ||
| Dermanyssinae (p. 471). | |||
| Heterostigmata (p. 471) | Tarsonemidae (p. 471). | ||
| Prostigmata (p. 471) | Bdellidae (p. 471). | ||
| Halacaridae (p. 472). | |||
| Hydrachnidae (p. 472). | |||
| Trombidiidae (p. 472) | Limnocharinae (p. 472). | ||
| Caeculinae (p. 472). | |||
| Tetranychinae (p. 472). | |||
| Cheyletinae (p. 473). | |||
| Erythraeinae (p. 473). | |||
| Trombidiinae (p. 473). | |||
| Notostigmata (p. 473) | Opilioacaridae (p. 473). | ||
CRUSTACEA
CHAPTER I
CRUSTACEA—GENERAL ORGANISATION
The Crustacea are almost exclusively aquatic animals, and they play a part in the waters of the world closely parallel to that which insects play on land. The majority are free-living, and gain their sustenance either as vegetable-feeders or by preying upon other animals, but a great number are scavengers, picking clean the carcasses and refuse that litter the ocean, just as maggots and other insects rid the land of its dead cumber. Similar to insects also is the great abundance of individuals which represent many of the species, especially in the colder seas, and the naturalist in the Arctic or Antarctic oceans has learnt to hang the carcasses of bears and seals over the side of the boat for a few days in order to have them picked absolutely clean by shoals of small Amphipods. It is said that these creatures, when crowded sufficiently, will even attack living fishes, and by sheer press of numbers impede their escape and devour them alive. Equally surprising are the shoals of minute Copepods which may discolour the ocean for many miles, an appearance well known to fishermen, who take profitable toll of the fishes that follow in their wake. Despite this massing together we look in vain for any elaborate social economy, or for the development of complex instincts among Crustacea, such as excite our admiration in many insects, and though many a crab or lobster is sufficiently uncanny in appearance to suggest unearthly wisdom, he keeps his intelligence rigidly to himself, encased in the impenetrable reserve of his armour and vindicated by the most powerful of pincers. It is chiefly in the variety of structure and in the multifarious phases of life-history that 4the interest of the Crustacea lies. Before entering into an examination of these matters, it will be well to take a general survey of Crustacean organisation, to consider the plan on which these animals are built, and the probable relation of this plan to others met with in the animal kingdom.
The Crustacea, to begin with, are a Class of the enormous Phylum Arthropoda, animals with metamerically segmented bodies and usually with externally jointed limbs. Their bodies are thus composed of a series of repeated segments, which are on the whole similar to one another, though particular segments may be differentiated in various respects for the performance of different functions. This segmentation is apparent externally, the surface of a Crustacean being divided typically into a number of hard chitinous rings, some of which may be fused rigidly together, as in the carapace of the crabs, or else articulated loosely.
Each segment bears typically a pair of jointed limbs, and though they vary greatly in accordance with the special functions for which they are employed, and may even be absent from certain segments, they may yet be reduced to a common plan and were, no doubt, originally present on all the segments.
Passing from the exterior to the interior of the body we find, generally speaking, that the chief system of organs which exhibits a similar repetition, or metameric segmentation, is the nervous system. This system is composed ideally of a nervous ganglion situated in each segment and giving off peripheral nerves, the several ganglia being connected together by a longitudinal cord. This ideal arrangement, though apparent during the embryonic development, becomes obscured to some extent in the adult owing to the concentration or fusion of ganglia in various parts of the body. The other internal organs do not show any clear signs of segmentation, either in the embryo or in the adult; the alimentary canal and its various diverticula lie in an unsegmented body-cavity, and are bathed in the blood which courses through a system of narrow canals and irregular spaces which surround all the organs of the body. A single pair, or at most two pairs of kidneys are present.
The type of segmentation exhibited by the Crustacea is thus of a limited character, concerning merely the external skin with its appendages, and the nervous system, and not touching any 5of the other internal organs.[1] In this respect the Crustacea agree with all the other Arthropods, in the adults of which the segmentation is confined to the exterior and to the nervous system, and does not extend to the body-cavity and its contained organs; and for the same reason they differ essentially from all other metamerically segmented animals, e.g. Annelids, in which the segmentation not only affects the exterior and the nervous system, but especially applies to the body-cavity, the musculature, the renal, and often the generative organs. The Crustacea also resemble the other Arthropoda in the fact that the body-cavity contains blood, and is therefore a “haemocoel,” while in the Annelids and Vertebrates the segmented body-cavity is distinct from the vascular system, and constitutes a true “coelom.” To this important distinction, and to its especial application to the Crustacea, we will return, but first we may consider more narrowly the segmentation of the Crustacea and its main types of variation within the group. In order to determine the number of segments which compose any particular Crustacean we have clearly two criteria: first, the rings or somites of which the body is composed, and to each of which a pair of limbs must be originally ascribed; and, second, the nervous ganglia.
Around and behind the region of the mouth there is very little difficulty in determining the segments of the body, if we allow embryology to assist anatomy, but in front of the mouth the matter is not so easy.
In the Crustacea the moot point is whether we consider the paired eyes and first pair of antennae as true appendages belonging to two true segments, or whether they are structures sui generis, not homologous to the other limbs. With regard to the first antennae we are probably safe in assigning them to a true body-segment, since in some of the Entomostraca, e.g. Apus, the nerves which supply them spring, not from the brain as in more highly specialised forms, but from the commissures which pass round the oesophagus to connect the dorsally lying brain to the ventral nerve-cord. The paired eyes are always innervated from the brain, but the brain, or at least part of it, is very 6probably formed of paired trunk-ganglia which have fused into a common cerebral mass; and the fact that under certain circumstances the stalked eye of Decapods when excised with its peripheral ganglion[2] can regenerate in the form of an antenna, is perhaps evidence that the lateral eyes are borne on what were once a pair of true appendages.
Now, with regard to the segmentation of the body, the Crustacea fall into three categories: the Entomostraca, in which the number of segments is indefinite; the Malacostraca, in which we may count nineteen segments, exclusive of the terminal piece or telson and omitting the lateral eyes; and the Leptostraca, including the single recent genus Nebalia, in which the segmentation of head and thorax agrees exactly with that of the Malacostraca, but in the abdomen there are two additional segments.
It has been usually held that the indefinite number of segments characteristic of the Entomostraca, and especially the indefinitely large number of segments characteristic of such Phyllopods as Apus, preserves the ancestral condition from which the definite number found in the Malacostraca has been derived; but recently it has been clearly pointed out by Professor Carpenter[3] that the number of segments found in the Malacostraca and Leptostraca corresponds with extraordinary exactitude to the number determined as typical in all the other orders of Arthropoda. This remarkable correspondence (it can hardly be coincidence) seems to point to a common Arthropodan plan of segmentation, lying at the very root of the phyletic tree; and if this is so, we are forced to the conclusion that the Malacostraca have retained the primitive type of segmentation in far greater perfection than the Entomostraca, in some of which many segments have been added, e.g. Phyllopoda, while in others segments have been suppressed, e.g. Cladocera, Ostracoda. It may be objected to this view of the primitive condition of segmentation in the Crustacea that the Trilobites, which for various reasons are regarded as related to the ancestral Crustaceans, exhibit an indefinite and often very high number of segments; but, as Professor Carpenter has pointed out, the oldest and most primitive of Trilobites, such as Olenellus, possessed 7few segments which increase as we pass from Cambrian to Carboniferous genera.
The following table shows the segmentation of the body in the Malacostraca, as compared with that of Limulus (cf. p. 263), Insecta, the primitive Myriapod Scolopendrella, and Peripatus. It will be seen that the correspondence, though not exact, is very close, especially in the first four columns, the number of segments in Peripatus being very variable in the different species.
| Table showing the Segmentation of various Arthropods | |||||
|---|---|---|---|---|---|
| Malacostraca. | Limulus. | Insecta. | Myriapoda. (Scolopendrella). | Peripatus. | |
| 1 | Eyes | Median eyes | Eyes | ||
| 2 | 1st antennae | Rostrum | Antennae | Feelers | Feelers |
| 3 | 2nd antennae | Chelicerae | Intercalary segment | ||
| 4 | Mandibles | Pedipalpi | Mandibles | Mandibles | Mandibles |
| 5 | 1st maxillae | 1st walking legs | Maxillulae | Maxillulae | 1st jaw-claw |
| 6 | 2nd maxillae | 2nd „ „ | 1st maxillae | 1st maxillae | 2nd jaw-claw |
| 7 | 1st maxillipede | 3rd „ „ | 2nd maxillae | 2nd maxillae | 1st leg |
| 8 | 2nd maxillipede | 4th „ „ | 1st leg | 1st leg | 2nd „ |
| 9 | 3rd maxillipede | Chilaria | 2nd „ | 2nd „ | 3rd „ |
| 10 | 1st ambulatory | Genital operculum | 3rd „ | 3rd „ | 4th „ |
| 11 | 2nd „ | 1st gill-book | 1st abdominal | 4th „ | 5th „ |
| 12 | 3rd „ | 2nd „ | 2nd „ | 6th „ | 6th „ |
| 13 | 4th „ | 3rd „ | 3rd „ | 6th „ | 7th „ |
| 14 | 5th „ | 4th „ | 4th „ | 7th „ | 8th „ |
| 15 | 1st abdominal | 5th „ | 5th „ | 8th „ | 9th „ |
| 16 | 2nd „ | No appendages | 6th „ | 9th „ | 10th „ |
| 17 | 3rd „ | „ | 7th „ | 10th „ | 11th „ |
| 18 | 4th „ | „ | 8th „ | 11th „ | 12th „ |
| 19 | 5th „ | „ | 9th „ | 12th „ | 13th „ |
| 20 | 6th „ | „ | 10th „ | Reduced limbs | 14th „ |
| 21 | [4] | „ | Cercopods | [5] | |
| Telson | Telson | Telson | Telson | Telson | |
The appendages of the Crustacea exhibit a wonderful variety 8of structure, but these variations can be reduced to at most two, and possibly to one fundamental plan. In a typical Crustacean, besides the paired eyes, which may be borne on stalks, possibly homologous to highly modified limbs, there are present, first, two pairs of rod-like or filamentous antennae, which in the adult are usually specialised for sensory purposes, but frequently retain their primitive function as locomotory limbs even in the adult, e.g. Ostracoda; while in the Nauplius larva, found in almost all the chief subdivisions of the Crustacea, the two pairs of antennae invariably aid in locomotion, and the base of the second antennae is usually furnished with sharp biting spines which assist mastication. Following the antennae is a pair of mandibles which are fashioned for biting the food or for piercing the prey, and posterior to these are two pairs of maxillae, biting organs more slightly built than the mandibles, whose function it is to lacerate the food and prepare it for the more drastic action of the mandibles. So far, with comparatively few exceptions, the order of specialisation is invariable; but behind the maxillae the trunk-appendages vary greatly both in structure and function in the different groups.
As a general rule, the first or first few thoracic limbs are turned forwards toward the mouth, and are subsidiary to mastication; they are then called maxillipedes; this happens usually in the Malacostraca, but to a much less extent in the Entomostraca; and in any case these appendages immediately behind the maxillae never depart to any great extent from a limb-like structure, and they may graduate insensibly into the ordinary trunk-appendages. The latter show great diversity in the different Crustacean groups, according as the animals lead a natatory, creeping, or parasitic method of life; they may be foliaceous, as in the Branchiopoda, or biramous, as in the swimming thoracic and abdominal appendages of the Mysidae, or simply uniramous, as in the walking legs of the higher Decapoda, and the clinging legs of various parasitic forms.
Without going into detailed deviations of structure, many of which will be described under the headings of special groups, it is clear from the foregoing description and from Fig. 1 (p. 10), that three main types of appendage can be distinguished: first, the foliaceous or multiramous; second, the biramous; and, third, the uniramous.
9We may dismiss the uniramous type with a few words: it is obviously secondarily derived from the biramous type; this can be proved in detail in nearly every case. Thus, the uniramous second antennae of some adult forms are during the Nauplius stage invariably biramous, a condition which is retained in the adult Cladocera. Similarly the uniramous walking legs of many Decapoda pass through a biramous stage during development, the outer branches or exopodites of the limbs being suppressed subsequently, while the primitively biramous condition of the thoracic limbs is retained in the adults of the Schizopoda, which doubtless own a common ancestry with the Decapoda. The only Crustacean limb which appears to be constantly uniramous both in larval and adult life is the first pair of antennae.
We are reduced, therefore, to two types—the foliaceous and biramous. Sir E. Ray Lankester,[6] in one of his most incisive morphological essays, has explained how these two types are really fundamentally the same. He compares, for instance, the foliaceous first maxillipede (Fig. 1, A), or the second maxilla (Fig. 1, B) of a Decapod, e.g. Astacus, with the foliaceous thoracic limb of Branchipus (Fig. 1, D), and with the typically biramous first maxillipede of a Schizopod (Fig. 1, F).
In each case there is present, on the outer edge of the limb, one or more projections or epipodites which are generally specialised for respiratory purposes, and may carry the gills. The 6th and 5th “endites” in the foliaceous limb (Fig. 1, D) are compared with the exopodite and endopodite respectively of the biramous limb, while the endites 4–1 of the foliaceous limb are found in the basal joints of the biramous limb. Lankester presumes that the biramous type of limb throughout has been derived from the foliaceous type by the suppression of the endites 1–4, as discrete rami, and the exaggerated development of the endites 5 and 6, as above indicated.

Fig. 1.—Appendages of Crustacea (A-G) and Trilobita (H). A, First maxillipede of Astacus; B, second maxilla of Astacus; C, second walking leg of Astacus; D, thoracic limb of Branchipus; E, first maxillipede of Mysis; F, first maxillipede of Gnathophausia; G, thoracic limb of Nebalia; H, thoracic limb of Triarthrus. bp, basipodite; br, bract; cp, carpopodite; cxp, coxopodite; cx.s, coxopoditic setae; dp, dactylopodite; end, endopodite; ep, epipodite; ex, exopodite; ip, ischiopodite; mp, meropodite; pp, propodite; 1–6, the six endites.
The essential fact that the two types of limb are built on the same plan may be considered as established; but it may be urged that the biramous type represents this common plan more nearly than the foliaceous. It is, at any rate, certain that in the maxillipedes of the Decapoda we witness the conversion of the biramous type into the foliaceous by the expansion of the basal joints concomitantly with the assumption by the maxillipedes of masticatory functions. Thus in the Decapoda the first maxillipede is decidedly foliaceous owing to the expanded “gnathobases” (Fig. 1, A, bp, cxp), and the second maxillipedes are flattened, with their basal joints somewhat expanded and furnished with biting hairs; but in the “Schizopoda” 11(e.g. Mysis) the first maxillipede is a typical biramous limb, though the expanded gnathobases in some forms are beginning to project (Fig. 1, E), while the limb following, which corresponds to the second maxillipede of Decapods, is simply a biramous swimming leg. Besides this obvious conversion of a biramous into a foliaceous limb, further evidence of the fundamental character of the biramous type is found, first, in its invariable occurrence in the Nauplius stage, which does not necessarily mean that the ancestors of the Crustacea possessed this type of limb in the adult, but which does imply that this type of limb was possessed at some period of life by the common ancestral Crustacean; and, second, the limbs of the Trilobita, a group which probably stands near the origin of the Crustacea, have been shown by Beecher to conform to the biramous type (Fig. 1, H). Furthermore, the thoracic limbs of Nebalia, an animal which combines many of the characteristics of Entomostraca and Malacostraca, and is therefore considered as a primitive type, despite their flattened character, are really built upon a biramous plan (Fig. 1, G).
In conclusion, we may point out that this view of the Crustacean limb, as essentially a biramous structure, agrees with the conclusion derived from our consideration of the segmentation of the body, and points less to the Branchiopoda as primitive Crustacea and more to some generalised Malacostracan type.
So far we have shortly dealt with those systems of organs which are clearly affected by the metameric segmentation of the body; we must now expose the condition of the body-cavity to a similar scrutiny. If we remove the external integument of a Crustacean, we find that the internal organs do not lie in a spacious and discrete body-cavity, as is the case in the Annelids and Vertebrates, but that they are packed together in an irregular system of spaces (“haemocoel”) in communication with the vascular system and containing blood. In the Entomostraca and smaller forms generally, a definite vascular system hardly exists, though a central heart and artery may serve to propel the blood through the irregular lacunae of the body-cavity; but in the larger Malacostraca a complicated system of arteries may be present which pour the blood into fairly definitely arranged spaces surrounding the chief organs. These spaces return the 12blood to the pericardium, and so to the heart again through the apertures or ostia which pierce its walls.
This condition of the body-cavity or haemocoel is reproduced in the adults of all Arthropods, but in some of them by following the development we can trace the steps by which the true coelom is replaced by the haemocoel. In the embryos of all Arthropods except the Crustacea, a true closed metamerically segmented coelom is formed as a split in the mesodermal embryonic layer of cells, distinct from the vascular system. During the course of development the segmented coelomic spaces and their walls give rise to the reproductive organs and to certain renal organs in Peripatus, Myriapoda, and Arachnida (nephridia and coxal glands), but the general body-cavity is formed as an extension of the vascular system, which is laid down outside the coelom by a canaliculisation of the extra-coelomic mesoderm. In the embryos of the Crustacea, however, there is never at any time a closed segmented coelom, and in this respect the Crustacea differ from all other Arthropods. The only clear instance in which metamerically repeated mesodermal cavities have been seen in the embryo Crustacean is that of Astacus; here Reichenbach[7] states that in the abdomen segmental cavities are formed which subsequently break down; but even in this instance no connexion has been shown to subsist between these embryonic cavities and the reproductive and excretory organs of the adult.
Since the connexion between the coelom and the excretory organs is always a very close one throughout the animal kingdom, interest naturally centres upon the renal organs in Crustacea, and it has been suggested that these organs in Crustacea represent the sole remains, with the possible exception of the gonads, of the coelom. Since, at any rate, a part of the kidneys appears to be developed as a closed sac in the mesoderm, and since they possess a possible segmental value, this suggestion is plausible; but, on the other hand, since there are never more than two pairs of kidneys, and since they are totally unconnected with the gonads or with any other indication of a segmented coelom, the suggestion remains purely hypothetical.
The renal organs of the Crustacea, excluding the Malpighian tubes present in some Amphipods which open into the alimentary canal, and resemble the Malpighian tubes of Insects, consist of 13two pairs—the antennary gland, opening at the base of the second antenna, and the maxillary gland, opening on the second maxilla. These two pairs of glands rarely subsist together in the adult condition, though this is said to be the case in Nebalia and possibly Mysis; the antennary glands are characteristic of adult Malacostraca[8] and the larvae of the Entomostraca, while the maxillary glands (“shell-glands”) are present in adult Entomostraca and larval Malacostraca, that is to say, the one pair replaces the other in the two great subdivisions of the Crustacea. The shell-gland of the Entomostraca is a simple structure consisting of a coiled tube opening to the exterior on the external branch of the second maxilla, and ending blindly in a dilated vesicle, the end-sac. The antennary gland of the Malacostraca is usually more complicated: these complications have been studied especially by Weldon,[9] Allen, and Marchal[10] in the Decapoda. In a number of forms we have a tube opening to the exterior at the base of the second antenna, and expanding within to form a spacious bladder into which the coiled tubular part of the kidney opens, while at the extremity of this coiled portion is the vesicle called the end-sac. This arrangement may be modified; thus in Palaemon Weldon described the two glands as fusing together above and below the oesophagus, the dorsal commissure expanding into a huge sac stretching dorsally down the length of the body. This closed sac with excretory functions thus comes to resemble a coelomic cavity, and the view that it is really coelomic has indeed been upheld.
A modified form of this view is that of Vejdovský, who describes a funnel-apparatus leading from the coiled tube into the end-sac of the antennary gland of Amphipods; he regards the end-sac alone as representing the coelom, while the funnel and coiled tube represent the kidney opening into it.
Not very much is known of the development of these various structures. Some authors have considered that both antennary and maxillary glands are developed in the embryo from ectodermal inpushings, but the more recent observations of Waite[11] on Homarus americanus indicate that the antennary gland at 14any rate is a composite structure, formed by an ectodermal ingrowth which meets a mesodermal strand, and from the latter are produced the end-sac and perhaps the tubular excretory portions of the gland with their derivatives.
With regard to the possible metameric repetition of the renal organs, it is of interest to note that by feeding Mysis and Nebalia on carmine, excretory glands of a simple character were observed by Metschnikoff situated at the bases of the thoracic limbs.
The alimentary canal of the Crustacea is a straight tube composed of three parts—a mid-gut derived from the endoderm of the embryo, and a fore- and hind-gut formed by ectodermal invaginations in the embryo which push into and fuse with the endodermal canal. The regions of the fore- and hind-gut can be recognised in the adult by the fact of their being lined with the chitinous investment which is continued over the external surface of the body forming the hard exoskeleton, while the mid-gut is naked. The chitinous lining of fore- and hind-gut is shed whenever the animal moults. In the Malacostraca, in which a complicated “gastric mill” may be present, the chitinous lining of this part of the gut is thrown into ridges bearing teeth, and this stomach in the crabs and lobsters reaches a high degree of complication and materially assists the mastication of the food. The gut is furnished with a number of secretory and metabolic glands; the so-called liver, which is probably a hepatopancreas, opening into the anterior end of the mid-gut, is directed forwards in most Entomostraca and backwards in the Malacostraca, in the Decapoda developing into a complicated branching organ which fills a large part of the thorax. In the Decapoda peculiar vermiform caeca of doubtful function are present, a pair of which open into the gut anteriorly where fore-passes into mid-gut, and a single asymmetrically placed caecum opens posteriorly into the alimentary tract where mid- passes into hind-gut.
The disposition of these caeca, marking as they do the morphological position of fore-, mid-, and hind-gut, is of peculiar interest owing to the variations exhibited. From some unpublished drawings of Mr. E. H. Schuster, which he kindly lent me, it appears that in certain Decapods, e.g. Callianassa subterranea, the length of the mid-gut between the anterior and posterior caeca is very long; in Carcinus maenas it is considerable; 15in Maia squinado it is greatly reduced, the caeca being closely approximated; while in Galathea strigosa the caeca are greatly reduced, and the mid-gut as a separate entity has almost disappeared. The relation of these variations to the habits of the different crabs and to their modes of development is unknown.
The reproductive organs usually make their appearance as a small paired group of mesodermal cells in the thorax comparatively late in life; and neither in their early development nor in the adult condition do they show any clear signs of segmentation or any connexion with a coelomic cavity. The sexes are usually separate, but hermaphroditism occurs sporadically in many forms, and as a normal condition in some parasitic groups (see pp. 105–107). The adult gonads are generally simple paired tubes, from the walls of which the germ-cells are produced, and as these grow and come to maturity they fill up the cavities of the tubes; special nutrient cells are rarely differentiated, though in some cases (e.g. Cladocera) a few ova nourish themselves by devouring their sister-cells (see p. 44). The oviducts and vasa deferentia are formed as simple outgrowths from the gonadial tubes, which acquire an opening to the exterior; they are usually poorly supplied with accessory glands, the epithelium of the canals often supplying albuminous secretions for cementing the eggs together, while the lining of the vasa deferentia may be instrumental in the formation of spermatophores for transferring large packets of spermatozoa to the female. In the vast majority of Crustacea copulation takes place, the male passing spermatophores or free spermatozoa into special receptacles (spermathecae), or into the oviducts of the female. The spermatophores are hollow chitinous structures in which the spermatozoa are packed; they are often very large and assume characteristic shapes, especially in the Decapoda.
The spermatozoa show a great variety of structure, but they conform to two chief types—the filiform, which are provided with a long whip-like flagellum; and the amoeboid, which are furnished with radiating pseudopodia, and are much slower in their movements. The amoeboid spermatozoa of some of the Decapoda contain in the cell-body a peculiar chitinous capsule, and Koltzoff[12] has observed that when the spermatozoon has 16settled upon the surface of the egg the chitinous capsule becomes suddenly exceedingly hygroscopic, swells up, and explodes, driving the head of the spermatozoon into the egg. We cannot enter here into a description of the embryological changes by which the egg is converted into the adult form. Crustacean eggs as a whole contain a large quantity of yolk, but in some forms total segmentation occurs in the early stages, which is converted later into the pyramidal type, i.e. the blastomeres are arranged round the edge, and the yolk in the centre is only partly segmented to correspond with them. The eggs during the early stages of development are in almost all cases (except Branchiura, p. 77, and Anaspides, p. 116) carried about by the female either in a brood-pouch (Branchiopoda, Ostracoda, Cirripedia, Phyllocarida, Peracarida), or agglutinated to the hind legs or some other part of the body (Copepoda, Eucarida), or in a chamber formed from the maxillipedes (Stomatopoda). Development may be direct, without a complicated metamorphosis, or indirect, the larva hatching out in a form totally different to the adult state, and attaining the latter by a series of transformations and moults. The various larval forms will be described under the headings of the several orders.
The respiratory organs are typically branchiae, i.e. branched filamentous or foliaceous processes of the body-surface through which the blood circulates, and is brought into close relation with the oxygen dissolved in the water. In most of the smaller Entomostraca no special branchiae are present, the interchange of gases taking place over the whole body-surface; but in the Malacostraca the gills may reach a high degree of specialisation. They are usually attached to the bases of the thoracic limbs (“podobranchiae”), to the body-wall at the bases of these limbs, often in two series (“arthrobranchiae”), and to the body-wall some way above the limb-articulations (“pleurobranchiae”). In an ideal scheme each thoracic appendage beginning with the first maxillipede would possess a podobranch, two arthrobranchs, and a pleurobranch, but the full complement of gills is never present, various members of the series being suppressed in the various orders, and thus giving rise to “branchial formulae” typical of the different groups.
After this brief survey of Crustacean organisation we 17may be able to form an opinion upon the position of the Crustacea relative to other Arthropoda, and upon the question debated some time ago in the pages of Natural Science[13] whether the Arthropoda constitute a natural group. The Crustacea plainly agree with all the other Arthropoda in the possession of a rigid exoskeleton segmented into a number of somites, in the possession of jointed appendages metamerically repeated, some of which are modified to act as jaws; they further agree in the general correspondence of the number of segments of which the body is primitively composed; the condition of the body-cavity or haemocoel is also similar in the adult state. An apparently fundamental difference is found in the entire absence during development of a segmented coelom, but since this organ breaks down and is much reduced in all adult Arthropods, it is not difficult to believe that its actual formation in the embryo as a distinct structure might have been secondarily suppressed in Crustacea.
The method of breathing by gills is paralleled by the respiratory structures found in Limulus and Scorpions; the transition, if it occurred, from branchiae to tracheae cannot, it is true, be traced, but the separation of Arthropods into phyletically distinct groups of Tracheata and Branchiata on this single characteristic is inadmissible. On the whole the Crustacea may be considered as Arthropods whose progenitors are to be sought for among the Trilobita, from whose near relations also probably sprang Limulus and the Arachnids.
CHAPTER II
CRUSTACEA (CONTINUED): ENTOMOSTRACA—BRANCHIOPODA—PHYLLOPODA—CLADOCERA—WATER-FLEAS
SUB-CLASS I.—ENTOMOSTRACA.
The Entomostraca are mostly small Crustacea in which the segmentation of the body behind the head is very variable, both in regard to the number of segments and the kind of differentiation exhibited by those segments and their appendages. An unpaired simple eye, known as the Nauplius eye from its universal presence in that larval form, often persists in the adult, and though lateral compound eyes may be present they are rarely borne on movable stalks. In the adult the excretory gland (“shell-gland”) opens on the second maxillary segment, but in the larval state or early stages of development a second antennary gland may also be present, which disappears in the adult. The liver usually points forwards, and is simple and saccular in structure, and the stomach is not complicated by the formation of a gastric mill. With the exception of most Cladocera and Ostracoda the young hatch out in the Nauplius state.
Order I. Branchiopoda.[14]
The Branchiopods are of small or moderate size, with flattened and lobate post-cephalic limbs, and with functional gnathobases. Median and lateral eyes are nearly always present. The labrum is large, and the second maxillae are small or absent in the adult.
Branchiopods are found in every part of the world; a few are marine, but the great majority are confined to inland lakes and ponds, or to slowly-moving streams. The fresh waters, from the 19smallest pools to the largest lakes, often swarm with them, as do those streams which flow so slowly that the creatures can obtain occasional shelter among vegetation along the sides and bottom without being swept away, while even rivers of considerable swiftness contain some Cladocera. Several Branchiopods are found in the brackish waters of estuaries, and some occur in lakes and pools so salt that no other Crustacea, and few other animals of any kind, can live in them. The great majority swim about with the back downwards, collecting food in the ventral groove between their post-oral limbs, and driving it forwards, towards the mouth, by movements of the gnathobases (p. 10). The food collected in this way consists largely of suspended organic mud, together with Diatoms and other Algae, and Infusoria; the larger kinds, however, are capable of gnawing objects of considerable size, Apus being said to nibble the softer insect larvae, and even tadpoles. Many Cladocera (e.g. Daphnia, Simocephalus) may be seen to sink to the bottom of an aquarium, with the ventral surface downwards, and to collect mud, or even to devour the dead bodies of their fellows, while Leptodora is said to feed upon living Copepods, which it catches by means of its antennae.
The Branchiopoda fall naturally into two Sub-orders, the Phyllopoda including a series of long-bodied forms, with at least ten pairs of post-cephalic limbs, and the Cladocera with shorter bodies and not more than six pairs of post-cephalic limbs.
Sub-Order 1. Phyllopoda.
The Phyllopoda include a series of genera which differ greatly in appearance, owing to differences in the development of the carapace, which are curiously correlated with differences in the position of the eyes. Except in these points, the three families which the sub-order contains are so much alike that they may conveniently be described together.
In the Branchipodidae the carapace is practically absent, being represented only by the slight backward projection on each side of the head which contains the kidney (Fig. 2); the paired eyes are supported on mobile stalks, and project freely, one on either side of the head.
In the Apodidae[15] the head is broad and depressed, the ventral 20side being nearly flat, the dorsal surface convex; the hinder margin of the head is indicated dorsally by a transverse cervical ridge, bounded by two grooves, behind which the carapace projects backwards as a great shield, covering at least half the body, but attached only to the back of the head. In Lepidurus productus the head and carapace together form an oval expansion, deeply emarginate at the hinder, narrower end, the sides of the emargination being toothed. The carapace has a strong median keel. The kidneys project into the space between the folds of skin which form the carapace, and their coils can be seen on each side, the terminal part of each kidney-tube entering the head to open at the base of the second maxilla. In all Branchiopoda with a well-developed carapace the kidney is enclosed in it in this way, whence the older anatomists speak of it as the “shell-gland.”

Fig. 2.—Chirocephalus diaphanus, female, × 5, Sussex. D.O, Dorsal organ; H, heart; Ov, ovary; U, uterus; V, external generative opening.
Associated with the development of the carapace, in this and in the next family, is a remarkable condition of the lateral eyes, which are sessile on the dorsal surface of the head, and near the middle line, the median eye being slightly in front of them. During embryonic life a fold of skin grows over all three eyes, so that a chamber is formed over them, which communicates with the exterior by a small pore in front.
In the Limnadiidae the body is laterally compressed, and the carapace is so large that at least the post-cephalic part of the body, and generally the head also, can be enclosed within it.

Fig. 3.—Limnetis brachyura, × 15. (After G. O. Sars.)
In Limnetis (Fig. 3) the dorsal surface of the head is bent downwards and is much compressed, the carapace being attached 21to it only for a short distance near the dorsal middle line. The sides of the carapace are bent downwards, and their margins can be pulled together by a transverse adductor muscle, so that the whole structure forms an ovoid or spheroidal case, from which the head projects in front, while the rest of the body is entirely contained within it. When the adductor muscle is relaxed the edges of the carapace gape slightly, like the valves of a Lamellibranch shell, and food-particles are drawn through the opening thus formed into the ventral groove by the movements of the thoracic feet, locomotion being chiefly effected by the rowing action of the second antennae, as in the Cladocera, to which all the Limnadiidae present strong resemblances in their method of locomotion, in the condition of the carapace, and in the form of the telson.
In Limnadia and Estheria the carapace projects not only backwards from the point of attachment to the head, but also forwards, so that the head can be enclosed by it, together with the rest of the body.
In all these genera the carapace is flexible along the middle dorsal line; in Estheria especially the softening of the dorsal cuticle goes so far that a definite hinge-line is formed, and this, together with the deposition of the lateral cuticle in lines concentrically arranged round a projecting umbo, gives the carapace a strong superficial likeness to a Lamellibranch shell, for which it is said to be frequently mistaken by collectors.
The eyes of the Limnadiidae are enclosed in a chamber formed by a growth of skin over them, as in Apodidae, but the pore by which this chamber communicates with the exterior is even more minute than in Apus. The paired eyes are so close together that they may touch (Limnadia, Estheria) or fuse (Limnetis); they are farther back than in the Apodidae, while the ventral curvature of the head causes the median eye to lie below them. In all 22these points the eyes of the Limnadiidae are intermediate between those of Apus and those of the Cladocera.
Dorsal Organ.—A structure very characteristic of adult Phyllopods is the “dorsal organ” (Figs. 2, 5, D.O), whose function is in many cases obscure. It is always a patch of modified cephalic ectoderm, supplied by a nerve from the anterior ventral lobe of the brain on each side; but its characters, and apparent function, differ in different forms. In the Branchipodidae the dorsal organ is a circular patch, far forward on the surface of the head (Figs. 2, 5, D.O). Its cells are arranged in groups, which remind one of the retinulae in a compound eye; each cell contains a solid concretion, and the concretions of a group may be so placed as to look like a badly-formed rhabdom. Claus,[16] who first called attention to this structure in the Branchipodidae, regarded it as a sense-organ. In Apodidae the dorsal organ is an oval patch of columnar ectoderm, immediately behind the eyes; it is slightly raised above the surrounding skin, and is covered by a very delicate cuticle (with an opening to the exterior?), and below it is a mass of connective tissue permeated by blood; Bernard has suggested that it is an excretory organ.
Most Limnadiidae resemble the Cladocera in the possession of a “dorsal organ” quite distinct from the above; in Limnetis and Estheria it has the form of a small pit, lined by an apparently glandular ectoderm, and this is its condition in many Cladocera; in Limnadia lenticularis it is a patch of glandular epithelium on a raised papilla. Limnadia has been observed to anchor itself to foreign objects by pressing its dorsal organ against them, and many Cladocera do the same thing; Sida crystallina, for example, will remain for hours attached by its dorsal organ to a waterweed or to the side of an aquarium. Structures resembling a dorsal organ occur in the larvae of many other Crustacea, but the presence of this organ in the adult is confined to Branchiopods, and indeed in many Cladocera it disappears before maturity. It is certain that the sensory and adhesive types of dorsal organ are not homologous, especially as rudimentary sense-organs may exist on the head of Cladocera together with the adhesive organ.
The telson differs considerably in the different genera. In the Branchipodidae[17] the anus opens directly backwards; and 23the telson carries two flattened backwardly directed plates, one on each side of the anus, the margins of each plate being fringed with plumose setae. In Artemia the anal plates are rarely as large as in Branchipus, and never have their margins completely fringed with setae; in A. salina from Western Europe, and in A. fertilis (Fig. 4, A) from the Great Salt Lake of Utah, there is a variable number of setae round the apical half of each lobe, but in specimens of A. salina from Western Siberia the number of setae may be very small, or they may be absent; in the closely allied A. urmiana from Persia the anal lobes are well developed in the male, each lobe bearing a single terminal hair, but they are altogether absent in the female. Schmankewitch and Bateson have shown that there is a certain relation between the salinity of the water in which Artemia salina occurs and the condition of the anal lobes, specimens from denser waters having on the whole fewer setae; the relation is, however, evidently very complex, and further evidence is wanted before any more definite statements can be made.

Fig. 4.—A, Ventral view of the anal region in Artemia fertilis, from the Great Salt Lake; B, ventral view of the telson and neighbouring parts of Lepidurus productus; C, side view of the telson and left anal lobe of Estheria (sp.?).
In the Apodidae the anal lobes have the form of two-jointed cirri, often of considerable length; in Apus the anus is terminal, but in Lepidurus (Fig. 4, B) the dorsal part of the telson is prolonged backwards, so as to form a plate, on the ventral face of which the anus opens, much as in the Malacostraca.
In the Limnadiidae (Fig. 4, C) the telson is laterally compressed 24and produced, on each side of the anus, into a flattened, upwardly curved process, sharply pointed posteriorly, and often serrate; the anal lobes are represented by two stout curved spines, while in place of the dorsal prolongation of Lepidurus we find two long plumose setae above the anus. In the characters of the telson and anal lobes, as in those of the head, the Limnadiidae approximate to the Cladocera. In Limnetis brachyura the ventral face of the telson is produced into a plate projecting backwards below the anus, in a manner which has no exact parallel among other Crustacea.
The appendages of the Phyllopoda are fairly uniform in character, except those affected by the sexual dimorphism, which is usually great.

Fig. 5.—Chirocephalus diaphanus, male. Side view of head, showing the large second antenna, A2, with its appendage Ap, above which is seen the filiform first antenna; D.O, dorsal organ; E1, median eye.

Fig. 6.—Chirocephalus diaphanus. Second antenna of male, uncoiled.
Of the cephalic appendages, the first antennae are generally small, and are never biramous; in Branchipus and its allies they are simple unjointed rods, in some species of Artemia they are three-jointed, in Apus they are feebly divided into two joints, while in Estheria they are many-jointed. The second antennae are the principal organs of locomotion in the Limnadiidae, where they are large and biramous; in all other Phyllopoda they are uniramous in the female, being either unjointed triangular plates as in Chirocephalus (Fig. 2), or minute vestigial filaments as in Apus, in which genus Zaddach, Huxley, and Claus have all failed to find any trace of a second antenna in some females. In the male Branchipodidae the second antennae are modified to form claspers, by which the female is seized, the various degrees of complication which these claspers exhibit affording convenient generic characters. In Branchinecta each second antenna is a thick, three-jointed rod, the last joint forming a claw, while the second joint is serrate on its inner margin; in Branchipus the base is much thickened, and bears on its inner side a large filament (perhaps represented by the proximal tubercle of Branchinecta and Artemia), which looks like an extra antenna. In Streptocephalus the terminal joint of the antenna is bifid, and there is a basal filament like that of Branchipus; in Chirocephalus diaphanus (Figs. 5, 6) the main branch of the antenna consists of two large joints, the terminal joint being a strong claw with a serrated process at its base, while the proximal joint bears two appendages on its inner side; one of these is a small, subconical tubercle, the second is more complicated, consisting of a main stem and five outgrowths. The main stem is many-jointed and flexible, its basal joint being longer than the others, and bearing on its outer side a large, triangular, membranous appendage, and four soft cylindrical appendages, the main stem and its appendages being beset with curious tubercles, ending in short spines, whose structure is not understood. Except during the act of copulation this remarkable apparatus is coiled on the inner side of the antennary claw, the jointed stem being so coiled that it is often compared to the 26coiled proboscis of a butterfly, and the triangular membrane folded like a fan beside it, so that much of the organ is concealed, and the general appearance of the head is that shown in Fig. 5. During copulation, the whole structure is widely extended.

Fig. 7.—Artemia fertilis. Front view of the head of a male, showing the large second antennae, A.2; A.1, first antennae.
The males of Artemia (Fig. 7) have the second antenna two-jointed, the basal joint bearing an inner tubercle, the terminal joint being flattened and bluntly pointed, its outer margin provided with a membranous outgrowth. In A. fertilis the breadth of the second joint varies greatly, the narrower forms presenting a certain remote resemblance to Branchinecta. In the males of Polyartemia the second antennae have a remarkable branched form not easily comparable with that found in other Branchipodidae.
The cephalic jaws are fairly uniform throughout the order. The mandibles have an undivided molar surface, and no palp; the first maxilla is very generally a triangular plate, with a setose biting edge; mandibles and maxillae are covered by the labrum. The second maxilla generally lies outside the chamber formed by the labrum, and is a simple oval plate, with or without a special process for the duct of the kidney.
The thoracic limbs, in front of the genital segments, are not as a rule differentiated into anterior maxillipedes and posterior locomotive appendages, as in higher forms; we have seen, however, that all these limbs take part in the prehension of food, and except in the Limnadiidae they all assist in locomotion. One of the middle thoracic legs of Artemia (Fig. 8, A) has a flattened stem, with seven processes on its inner, and two on its outer margin. The gnathobase (gn) is large, and fringed with long plumose setae, each of which is jointed; this is followed by four smaller “endites” (or processes on the median side), and then by two larger ones, the terminal endite (the sixth, 27excluding the gnathobase) being very mobile and attached to the main stem by a definite joint. On the outer side are two processes; a proximal “bract,” a flat plate with crenate edges, partly divided by a constriction into two, and a distal process, cylindrical and vascular, called by Sars and others the “epipodite.” In other Branchipodidae we have essentially the same condition, except that the fifth endite often becomes much larger than in Artemia, throwing the terminal endite well over to the outer edge of the limb; such a shift as this, continued farther, might well lead to the condition found in the Limnadiidae, or Apodidae, where the lobe which seems to represent the terminal endite of Artemia is entirely on the outer border of the limb, forming what most writers have called the exopodite (Lankester’s “flabellum”).[18] In the two last-named families the basal exite or bract of the Branchipodidae does not appear to be represented.

Fig. 8.—A, Thoracic limb of Chirocephalus diaphanus; B, prehensile thoracic limb of male Estheria. gn, Gnathobase; 1–6, the more distal endites.
The limbs of the Apodidae are remarkable in two ways; those in front of the genital opening (very constantly ten pairs) 28are not so nearly alike as in most genera of the sub-order, the first two pairs especially having the axis definitely jointed, while the endites are elongated and antenniform; further, while the first eleven segments bear each a single pair of limbs, as is usual among Crustacea, many of the post-genital segments bear several pairs; thus in Apus cancriformis there are thirty-two post-cephalic segments in front of the telson, the first eleven having each one pair of limbs, while the next seventeen have fifty-two pairs between them, the last four segments having none.
In all the Phyllopoda some of the post-cephalic limbs are modified for reproductive purposes; in the Branchipodidae the last two pairs (the 12th and 13th generally, the 20th and 21st in Polyartemia) are so modified in both sexes. In the female these appendages fuse at an early period of larval life, and surround the median opening of the generative duct (Fig. 2); in the male the two pairs also fuse, but traces of the limbs are left as eversible processes round the paired openings of the vasa deferentia.
In the other families, one or more limbs of the female are adapted for carrying or supporting the eggs. In the Apodidae the appendages of the eleventh segment have the exopodite in the form of a rounded, watchglass-shaped plate, fitting over a similarly shaped process of the axis of the limb, so that a lens-shaped box is formed, into which the eggs pass from the oviduct. In Limnadiidae the eggs are carried in masses between the body and the carapace, and are kept in position by special elongations of the exopodites of two or three legs, either those near the middle of the thorax (Estheria, Limnadia), or at its posterior end (Limnetis). In female Limnetis the last thoracic segments bear two remarkable lateral plates, which apparently also help to support the eggs. In the male Limnadiidae, the first (Limnetis) or the first two thoracic feet (Limnadia, Estheria) are prehensile (Fig. 8, B).
Alimentary Canal.—The mouth of the Phyllopoda is overhung by the large labrum, so that a kind of atrium is formed, outside the mouth itself, in which mastication is performed; numerous unicellular glands, opening on the oral face of the labrum, pour their secretion into the atrial chamber, and may be called salivary, though the nature of their secretion is not known. The mouth has commonly two swollen and setose 29lips, running longitudinally forwards from the bases of the first maxillae, and often wrapping round the blades of the mandibles. It leads into a vertical oesophagus, which opens into a small globular stomach, lying entirely within the head; the terminal part of the oesophagus is slightly invaginated into the stomach, so that a valvular ring is formed at the junction of the two. The stomach opens widely behind into a straight intestine, which runs backwards to about the level of the telson, where it joins a short rectum, leading to the terminal or ventral anus. The stomach and intestine are lined by a columnar epithelium, and covered by a thin network of circularly arranged muscle-fibres; the rectum has a flatter epithelium, and radial muscles pass from it to the body-wall, so that it can be dilated. The only special digestive glands are two branched glandular tubes, situated entirely within the head, which open into the stomach by large ducts, one on each side. In Chirocephalus the gastric glands are fairly small and simple; in the Apodidae their branches are more complex and form a considerable mass, filling all that portion of the head which is not occupied by the nervous system and the muscles. Backwardly directed gastric glands, like those of the higher Crustacea, are not found in Branchiopods; both forms occur together in the genus Nebalia, but with this exception the forwardly-directed glands are peculiar to Branchiopods.
Heart.—In Branchipus and its allies, and in Artemia, the heart extends from the first thoracic segment to the penultimate segment of the body, and is provided with eighteen pairs of lateral openings, one pair in every segment through which it passes except the last; it is widely open at its hinder end, and is prolonged in front for a short distance as a cephalic aorta, the rest of the blood-spaces being lacunar.
In most, at least, of the other Branchiopods, the heart is closed behind and is shortened; in Apus and Lepidurus it only extends through the first eleven post-cephalic segments, while in the Limnadiidae it is shorter still, the heart of Limnetis passing through four segments only. In all cases there is a pair of lateral openings in every segment traversed by the heart.
The blood of the Branchipodidae and Apodidae contains dissolved haemoglobin, the quantity present being so small as to give but a faint colour to the blood in Branchipus, while 30Artemia has rather more, and the blood of Apus is very red. The only other Crustacea in which the blood contains haemoglobin are the Copepods of the genus Lernanthropus,[19] so that the appearance of this substance is as irregular and inexplicable in Crustacea as in Chaetopods and Molluscs.
The nervous system of Branchipus may be described as an illustration of the condition prevailing in the group. The brain consists of two closely united ganglia, in each of which three main regions may be distinguished; a ventral anterior lobe, a dorsal anterior lobe, and a posterior lobe. The ventral anterior lobes give off nerves to the median eye, to the dorsal organ, and to a pair of curious sense-organs, comparable with the larval sense-knobs of many higher forms, situated one on each side of the median eye; in late larvae Claus describes the terminal apparatus of each frontal sense-organ as a single large hypodermic cell; W. K. Spencer[20] has lately described several terminal cells, containing peculiar chitinous bodies, in the adult. The homologous sense-organs of Limnetis are apparently olfactory. The dorsal anterior lobes give off the large nerves to the lateral eyes, while the posterior lobes supply the first antennae. The oesophageal connectives have a coating of ganglion-cells, and some of these form the ganglion of the second antenna, the nerve to this appendage leaving the connective just behind the brain. The post-oral nerve-cords are widely separate, each of them dilating into a ganglion opposite every appendage, the two ganglia being connected by two transverse commissures. The ganglia of the three cephalic jaws, so often fused in the higher Crustacea, are here perfectly distinct. Closely connected with each thoracic ganglion is a remarkable unicellular gland, opening to the exterior near the middle ventral line; it is conceivable that these cells may be properly compared with the larval nephridia of a Chaetopod,[21] but no evidence in support of such a comparison has yet been adduced.
Behind the genital segments, where there are no limbs, the nerve-cords run backwards without dilating into segmental ganglia, except in the anterior two abdominal segments where 31small ganglionic enlargements occur. In Apodidae, on the other hand, those segments which carry more than one pair of appendages have as many pairs of ganglia, united by transverse commissures, as they have limbs.
A stomatogastric nervous system exists in Apus, where a nerve arises on each side from the first post-oral commissure, and runs forward to join its fellow of the opposite side on the anterior wall of the oesophagus. From the loop so formed a larger median and a series of smaller lateral nerves pass to the wall of the alimentary canal. A second nerve to the oesophagus is given off from the mandibular ganglion of each side.
Reproductive Organs.—In Chirocephalus the ovaries (Fig. 2, Ov) are hollow epithelial tubes, lying one on each side of the alimentary canal, and extending from the sixth abdominal segment forwards to the level of the genital opening; at this point the two ovaries are continuous with ducts, which bend sharply downwards and open into the single uterus contained within the projecting egg-pouch and opening to the exterior at the apex of that organ. Short diverticula of the walls of the uterus receive the ducts of groups of unicellular glands, the bodies of which contain a peculiar opaque secretion, said to form the eggshells. In Apodidae the ovaries are similar in structure, but they are much larger and branch in a complex manner, while each ovary opens to the exterior independently of the other in the eleventh post-cephalic segment; nothing like the median uterus of the Branchipodidae being formed. The epithelium of the ovarian tubes proliferates, and groups of cells are formed; one becoming an ovum, the others being nutrient cells like those which will be more fully described in the Cladocera.
In Chirocephalus the testes are tubes similar in shape and position to the ovaries, each communicating in front with a short vas deferens, which dilates into a vesicula seminalis on its way to the eversible penis; an essentially similar arrangement is found in all Branchipodidae, but in Apodidae and Limnadiidae there is no penis.
All the Branchiopoda are dioecious,[22] and many are parthenogenetic. Among Branchipodidae Artemia is the only genus known to be parthenogenetic, but parthenogenesis is common in 32all Apodidae, while the males of several species of Limnadia are still unknown, although the females are sometimes exceedingly common. In Artemia, generations in which the males are about as numerous as the females seem to alternate fairly quickly with others which contain only parthenogenetic females; in Apus males are rarely abundant, and often absent for long periods; during five consecutive years von Siebold failed to discover a male in a locality in Bavaria, though he examined many thousands of individuals; near Breslau he found on one occasion about 11 per cent of males (114 in 1026), but in a subsequent year he found less than 1 per cent; the greatest recorded percentage of males is that observed by Lubbock in 1863, when he found 33 males among 72 individuals taken near Rouen.
The eggs of most genera can resist prolonged periods of desiccation, and indeed it seems necessary for the development of many species that the eggs should be first dried and afterwards placed in water. Many eggs (e.g. of Chirocephalus diaphanus and Branchipus stagnalis) float when placed in water after desiccation, the development taking place at the surface of the water.
Habitat.—All the Phyllopoda, except Artemia, are confined to stagnant shallow waters, especially to such ponds as are formed during spring rains, and dry up during the summer. In waters of this kind the species of Branchipus, Apus, etc., develop rapidly, and produce great numbers of eggs, which are left in the dried mud at the bottom after evaporation of the water, where they remain quiescent until a fresh rainy season. The mud from the beds of such temporary pools often contains large numbers of eggs, which may be carried by wind, on the legs of birds, and by other means, to considerable distances. Many exotic species have been made known to European naturalists by their power of hatching out when mud brought home by travellers is placed in water. The water of stagnant pools quickly dissolves a certain quantity of solid matter from the soil, and often receives dissolved solids through surface drainage from the neighbouring land; such salts may remain as the water evaporates, so that the water which remains after evaporation has proceeded for some time may be very sensibly denser than that in which the Branchiopods were hatched; these creatures must therefore be able to endure a considerable increase in the salinity of the surrounding waters during 33the course of their lives. My friend Mr. W. W. Fisher points out that the plants present in such a pond would often precipitate the carbonate of lime, so that this might be removed as evaporation went on, but that chlorides would probably remain in solution; from analyses which Mr. Fisher has been kind enough to make for me, it is seen that this happened in a small aquarium in my laboratory, in which Chirocephalus diaphanus lived for four months. In April, mud from the dry bed of a pond, known to contain eggs of Chirocephalus, was placed in this aquarium in Oxford, and water was added from the tap. Oxford tap-water contains about 0·3 grm. salts per litre, the chlorine being equivalent to 0·023 grm. NaCl. Water was added from time to time during May and June, but in July evaporation was allowed to proceed unchecked. At the end of July there was about half the original volume of water, the Chirocephalus being still active; the residue contained 0·96 grm. dissolved solids per litre, with chlorine equal to 0·19 grm. NaCl, so that the percentage of chlorides was about eight times the initial percentage, but there were only three and a fifth times the original amount of total solid matter in solution, the carbonate of lime having precipitated as a visible film.
Some species of Branchipus (e.g. B. spinosus, M. Edw.) and of Estheria (E. macgillivrayi, Baird, E. gubernator, Klutzinger) occur in salt pools, but Artemia flourishes in waters beside whose salinity that endured by any other Branchiopod is insignificant. In the South of Europe, Artemia salina may be found in swarms, as it used to be found in Dorsetshire, in the shallow brine-pans from which salt is commercially prepared; Rathke quotes an analysis showing that a pool in the Crimea contained living Artemia when the salts in solution were 271 grms. per litre, and the water was said to have the colour and consistency of beer.
The behaviour of the animals in the water differs a little; in normal feeding all the species swim with the back downwards, as has already been said; the Branchipodidae rarely settle on the ground, or on foreign objects, but the Apodidae occasionally wriggle along the bottom on their ventral surface, and Estheria burrows in mud.
The greater number of species are found in pools in flat, low-lying regions, and many appear to be especially abundant near 34the sea; Apus cancriformis has, however, been found in Armenia at 10,000 feet above sea level.
Wells and underground waters do not generally contain Phyllopods; but a species of Branchipus and one of Limnetis, both blind, have been described from the caves of Carniola.
One of the many puzzles presented by these creatures is the erratic way in which they are scattered through the regions they inhabit; a single small pond, a few yards or less in diameter, may be the only place within many miles in which a given species can be found; in this pond it may, however, appear regularly season after season for some time, and then suddenly vanish.
Geographically, the Phyllopoda are cosmopolitan, representatives of every family and of some genera (e.g. Streptocephalus, Lepidurus, Estheria) being found in every one of the great zoological regions, though a few aberrant genera are of limited range, thus Polyartemia is known only from the northern Palaearctic and Nearctic regions, Thamnocephalus only from the Central United States. The genus Artemia is not at present known in Australia.[23] The only recorded British species are Chirocephalus diaphanus, Artemia salina, and Apus cancriformis,[24] but other continental islands, for example the West Indian group, are better supplied. The distribution of the species is very imperfectly known, but on the whole every main zoological region seems to have its own peculiar species, which do not pass beyond its boundaries. Branchinecta paludosa and Lepidurus glacialis are circumpolar, both occurring in Norway, in Lapland, in Greenland, and in Arctic North America; but with these exceptions the Palaearctic and Nearctic species seem to be distinct. The European species Apus cancriformis occurs in Algiers, but the relations between the species of Northern Africa as a whole and those of Southern Europe on the one hand, or of Central and Southern Africa on the other, have yet to be worked out.
The soft-bodied Branchipodidae are not known in the fossil condition;[25] an Apus, closely related to the modern A. cancriformis, has been found in the Trias, but the most numerous remains have been left, as might be expected, by the hard-shelled Limnadiidae; 35carapaces, closely resembling those of the modern Estheria, are known in beds of all ages from the Devonian period to recent times; these carapaces are in several cases associated with fossils of an apparently marine type. None of the fossil species differ in any important characters from those now living, so that the Phyllopoda have existed in practically their present form for an enormously long period; this fact, and the evidence that species of existing genera were at one time marine, explain the wide distribution of animals at present restricted to a remarkably limited range of environmental conditions.
Summary of the Characters of the Genera.
Sub-Order Phyllopoda.—Branchiopoda with an elongated body, provided with at least ten pairs of post-cephalic limbs, the heart extending through four or more thoracic segments, and having at least four pairs of ostia.
Fam. 1. Branchipodidae.[26]—Carapace rudimentary, eyes stalked; the second antennae flat and unjointed in the female, jointed and prehensile in the male; female generative opening single; telson not laterally compressed, bearing two flattened lobes, or none. The heart extending through the thorax and the greater part of the abdomen.
A. Eleven pairs of praegenital ambulatory limbs.
a. Abdomen of six well-formed segments and a telson; anal lobes well formed, their margins setose.
Branchinecta, Verrill—Second antennae of ♂ without lateral appendages; ovisac of ♀ elongated. B. paludosa, O. F. Müll.—Circumpolar.
Branchiopodopsis, G. O. Sars[27]—Second antennae of ♂ as in Branchinecta; ovisac of ♀ short. B. hodgsoni, G. O. Sars—Cape of Good Hope.
Branchipus, Schaeffer—Second antennae of ♂ with simple internal filamentous appendage. B. stagnalis, Linn.—Central Europe.
Streptocephalus, Baird—Second antennae of ♂ 3–jointed, the last joint bifid; an external filamentous appendage. S. torvicornis, Wagn., Poland.
Chirocephalus, Prévost—Second antennae of ♂ 3–jointed, with a jointed internal appendage, which bears secondary processes, four cylindrical and one lamellar. C. diaphanus, Prévost (Fig. 2, p. 20).—Britain, Central Europe.
b. Abdominal segments five or fewer, and a telson. Anal lobes small or 0, sparsely or not at all setose.
Artemia, Leach—Second antennae of ♂ without filamentous 36appendage, 2–jointed, the second joint lamellar. A. salina, Linn.—Brine pools of the Palaearctic region.
c. Hinder abdominal segments united with telson to form a fin; anal lobes absent.
Thamnocephalus, Packard—Head with a branched median process of unknown nature. Only species T. platyurus, Packard—Kansas, U.S.A.
B. Nineteen pairs of praegenital ambulatory limbs.
Polyartemia, Fischer—Second antennae of ♂ forcipate; ovisac of ♀ very short. Only species P. forcipata, Fisch.
Fam. 2. Apodidae.[28]—Carapace well developed as a depressed shield, covering at least half the body. Eyes sessile, covered; no male clasping organs; anal lobes long, jointed cirri.
Apus, Scopoli—Telson not produced backwards over the anus; endites of first thoracic limb very long. A. cancriformis, Schaeffer—Britain, Europe, Algiers, Tunis. A. australiensis, Central Australia.
Lepidurus, Leach—Telson produced backwards to form a plate above the anus; endites of first thoracic limb short. L. productus, Bosc.—Central Europe. L. viridis, Southern Australia, New Zealand, L. patagonicus, Bergh, Argentines.
Fam. 3. Limnadiidae.—Body compressed; carapace in the form of a bivalve shell, the two halves capable of adduction by means of a strong transverse muscle; second antennae biramous, alike in both sexes; in the male, the first or the first and second thoracic limbs prehensile; telson laterally compressed.
A. Only the first thoracic limbs prehensile in the male; the carapace spheroidal, without lines of growth; head not included within the carapace-chamber.
Limnetis, Lovén—Compound eyes fused; anal spines absent; ambulatory limbs 10–12. L. brachyura, O. F. Müll (Fig. 3, p. 21).—Norway, Central Europe.
B. The first and second thoracic limbs prehensile in the male; carapace distinctly bivalve, enclosing the head, with concentric lines of growth round a more or less prominent umbo.
Eulimnadia, Packard—Carapace narrowly ovate, with few (4–5) lines of growth. E. mauritani, Guérin—Mauritius. E. texana, Packard—Texas, Kansas.
Limnadia, Brongniart—Carapace broadly ovate, with numerous lines of growth, without distinct umbones; L. lenticularis, Linn.—Northern and Central Europe.
Estheria, Rüppell—Carapace with well-marked umbones and numerous lines of growth, oval; E. tetraceros, Kryneki—Central Europe.
Leptestheria,[29] G. O. Sars—Carapace compressed, oblong. Rostrum 37with a movable spine; thoracic limbs with accessory lappet on the exopodite. L. siliqua, G. O. Sars—Cape Town.
Cyclestheria,[30] G. O. Sars. C. hislopi, Baird—Queensland, India, East Africa, Brazil.
Sub-Order 2. Cladocera.
The Cladocera are short-bodied Branchiopods, with not more than six pairs of thoracic limbs. The second antennae are important organs of locomotion, and are nearly always biramous; the first antennae are small, at least in the female; the second maxillae are absent in the adult. The carapace may extend backwards so as to enclose the whole post-cephalic portion of the body, or may be reduced to a small dorsal brood-pouch, leaving the body uncovered.
The Cladocera or “Water-fleas” are never of great size; Leptodora hyalina, the largest, is only about 15 mm. long, while many Lynceidae are not more than 0·1 or 0·2 mm. in length.
The head is bent downwards in all the Cladocera, so that parts which are morphologically anterior, such as the median eye and the first antennae, lie ventral to or even behind the compound eyes and the second antennae (cf. Fig. 10).
The compound lateral eyes fuse at an early period of embryonic life, so that they form a single median mass in the adult, over which a fold of ectoderm grows, to make a chamber over the eye, like that found in the Limnadiidae, except that it is completely closed. The fused eyes are generally large and conspicuous; in some deep-water forms the retinular elements of the dorsal portion are larger than those of the ventral (e.g. Bythotrephes, Fig. 13). In one or two species which live at very great depths, or in caves, the eyes are altogether absent.
The appendages of the head are fairly uniform, the most variable being the first antennae. In the females of many genera the first antennae are short and immovable, consisting of a single joint, with a terminal bunch of sensory hairs, and often a long lateral hair, as in Simocephalus (Figs. 9, 10), Daphnia, etc. In the female Moina (Fig. 16) they are movable, as they are in Ceriodaphnia and some others; in Bosmina (Fig. 22) and many Lyncodaphniidae they are elongated and imperfectly divided 38into joints by rings of spines, while in Macrothrix they are flattened plates. In the males the first antennae are elongated and mobile (cf. Figs. 11, 19).

Fig. 9.—Simocephalus vetulus, female. Ventral view, without the carapace; A1, A2, first and second antennae; For, head; Md, mandible; Te, telson; I-IV, first to fourth thoracic appendages.
The second antennae, the chief organs of locomotion, are biramous in all genera except Holopedium; the number of joints in each ramus, and the number of the long plumose hairs with which they are provided, are remarkably constant in whole series of genera, and are therefore useful for purposes of classification. The creatures row themselves by quick strokes of these appendages, the movement being slow and irregular in the rounder forms, such as Simocephalus or Daphnia, rapid and well directed in such elongated lacustrine forms as Bythotrephes or Leptodora.
The mandibles have no palp; the first maxillae are very small, and the second maxillae are absent (Fig. 9).
The carapace varies very much. In most genera (the Calyptomera of Sars) it is a large, backwardly-projecting fold of skin, bent downwards at the sides so as to form a bivalve shell, enclosing the whole post-cephalic portion of the body, as in Simocephalus (Fig. 10). The eggs are laid into the space between the carapace and the dorsal part of the thorax, both the carapace and the thorax itself being often modified for their protection and nutrition. In a few forms, the Gymnomera of Sars, the carapace serves only as a brood-pouch, which is distended when eggs are laid, but collapses to an inconspicuous appendage at the back of the head when it is empty (e.g. Leptodora, Fig. 24, Bythotrephes, Fig. 13). In the Calyptomera the surface of the carapace is frequently provided with a series of ridges, which may be parallel, rarely branching, as in Simocephalus; or in two sets which cross nearly at right angles, as in Daphnia; or so arranged as to form a hexagonal pattern, as 39in Ceriodaphnia. In a few forms the whole surface is irregularly covered with spines or scales. The hinder edge of the carapace is often produced into a median dorsal spine (Daphnia, Fig. 19), or more rarely there are two spines, one at each ventro-lateral corner (Scapholeberis, Fig. 20).

Fig. 10.—Simocephalus vetulus, × 30. Side view of female, showing the arrangement of the principal organs. A.2, Second antenna; C.S, cervical suture; E, fused compound eyes; H, heart; L, forwardly-directed gastric caeca; N, dorsal organ.
The cuticle of the carapace is often separated from that of the head by a cervical suture, as in Simocephalus (Fig. 10, C.S.) and near the line of demarcation many forms exhibit patches of glandular ectoderm which seem to be homologous with the dorsal adhesive organs of the Limnadiidae. The commonest condition is that of a median dorsal pit (Fig. 10, N.) by means of which the animal can fix itself to foreign objects. Certain forms may remain for long periods of time attached by the dorsal organ to plants, or to the sides of an aquarium, the only movement being a slow vibration of the feet, by which a current of water, sufficiently rapid for respiratory purposes, is established round it.[31] In Sida crystallina (Fig. 11) the dorsal organ is represented by three structures; in front there is a median raised 40patch (N.m) of columnar ectoderm, containing concretions like those described in the Branchipodidae, and behind this is a pair of cup-shaped organs (N.e), with raised margins.

Fig. 11.—Sida crystallina, male, × 27. Oxford. A.1, Elongated first antenna; N.e, paired element of dorsal organ; N.m, median element of dorsal organ; Te, testes; ♂, opening of vas deferens.
The fold of skin which forms the carapace contains the coils of the single pair of kidneys, and it forms an important organ of respiration, partly from the great size of the blood-vessels it contains, and partly from the presence of red, blue, or brown respiratory pigments in the tissue of the skin itself.
In most Cladocera the cuticle of the carapace is cast at every ecdysis, with that of other parts of the body; but in Iliocryptus and a few others it remains after each moult, giving the carapace an appearance of “lines of growth,” like that seen in many Limnadiidae.
The segmentation of the body behind the head is obscure, but we can generally recognise (1) a thorax, of as many segments as there are pairs of limbs; (2) an abdomen of three segments; and (3) a telson.
The thoracic limbs of the Calyptomera are flattened, and resemble those of the Phyllopoda; as a type we may examine the third thoracic limb of Simocephalus (Fig. 12, C), in which the axis bears a large setose gnathobase (Gn) on its inner edge, followed by two small endites; the terminal process, or exopodite (Ex) is a large flattened plate, with six long plumose hairs on its edge. The outer margin of the axis bears a bract (Br) and an epipodite.
In Simocephalus, as in the other Daphniidae, there are five pairs of thoracic limbs, of which the third and fourth are alike; in the female each limb of the first pair consists of a jointed axis, with strong biting hairs on the inner border, and a rudimentary epipodite (Fig. 12, A), the second limb being more like the third, but with a more prominent gnathobase and a narrower exopodite (B), while the limbs of the fifth pair have the gnathobase and the exopodite filamentous (D).
41In the Sididae there are six pairs of thoracic limbs, which are nearly alike in the female; in the Bosminidae there are six pairs, the first two modified for prehension, the last much reduced.

Fig. 12.—Thoracic limbs of female Simocephalus vetulus. A, The first; B, the second; C, the third; D, the fifth. Br, Bract; Ep, epipodite; Ex, exopodite; Gn, gnathobase.
In the male, the first thoracic limb is usually provided with a long sensory process and a prehensible hook (Figs. 11, 19).
In the Gymnomera the limbs are cylindrical, jointed rods, 42with a gnathobase on the inner side in the Polyphemidae, but not in Leptodora. The number varies from four to six pairs.
The abdomen bears no appendages. The telson is compressed in the Calyptomera, and is produced into two flattened plates, one on each side of the anal opening. The backwardly directed margins of these plates are commonly serrated, and the lower corner of each is produced into a curved spine, which carries secondary teeth. The number and arrangement of these teeth, though often extremely variable in the same species, are used extensively as specific characters. Above the anus the telson commonly bears two long plumose hairs, which are directed backwards.

Fig. 13.—Bythotrephes cederströmii, female, × 20, North Wales, from a specimen found by A. D. Darbishire. Car, carapace.
In the Gymnomera the telson is not bilaterally compressed, and it may be produced into a long spine, dorsal to the anus (e.g. Bythotrephes, Fig. 13).
The alimentary canal is extremely simple. The labrum is large, and forms a chamber above the mouth, into which food is driven by the limbs, as in the Phyllopoda, food being taken while the animal swims or lies on its back. The oesophagus runs vertically to join a small stomach, which bends sharply backwards and passes gradually into an intestine. In the last segment of the abdomen the intestine joins a short, thin-walled rectum, provided with radial muscles, by means of which it can be dilated. The dilatation of the rectum leads to an inhalation of water through the anus, which may possibly serve as a means of respiration. In the Daphniidae and Bosminidae there are two forwardly-directed digestive glands which open into the stomach, and in Eurycercus there is a large caecum at the junction of the rectum with the intestine. The 43intestine is usually straight, but in Lynceidae and in some Lyncodaphniidae it is coiled (e.g. Peracantha, Fig. 14).
In Leptodora the alimentary canal is altogether remarkable; the oesophagus is a long and very narrow tube, which runs back through the whole length of the thorax and joins the mid-gut in the third abdominal segment. The mid-gut is not differentiated into stomach and intestine; it has no diverticula of any kind, and runs straight backwards to join the short rectum a little in front of the anus.

Fig. 14.—Peracantha truncata, female, × 100. Oxford.
The heart is always short, and never has more than a single pair of lateral openings; it is longest in the Sididae, which show some approximation to the Phyllopods in this, as in the slight degree of difference between their anterior and posterior thoracic limbs. The pericardium lies in the one or two anterior thoracic segments, dorsal to the gut. From the heart the blood runs forwards to the dorsal part of the head, and passes backwards by three main channels, one entering each side of the carapace, while the third runs down the body, beneath the alimentary canal to dilate into a large sinus round the rectum. This ventral blood-channel gives a branch to each limb, which forms a considerable dilatation in the epipodite, the blood from the limb returning to the pericardium by a lateral sinus. From the rectum a large sinus runs forwards to the pericardium along the dorsal wall of the body. The blood which enters each half of the carapace is collected in a median vessel and returned through this to the pericardium.
Those spaces between the viscera which are not filled with blood are occupied by a peculiar connective tissue, consisting of rounded or polyhedral cells, charged with drops of a fatty material which is often brightly coloured.
The reproductive organs are interesting because of the peculiar phenomena connected with the nutrition of the two kinds of eggs. The ovaries or testes are epithelial sacs, one on 44each side of the body, each continuous with a duct which opens to the exterior behind the last thoracic limb. In the female, the opening is dorsal (Fig. 10), in the male it is ventral (Fig. 11). The external opening is usually simple; but in the male there is sometimes a penis-like process, on which the vas deferens opens (Daphnella).
The eggs are of two kinds, the so-called “summer-eggs,” with relatively little yolk, which develop rapidly without fertilisation, and the so-called “winter-eggs,” containing much yolk, which require to be fertilised and then develop slowly.
At one end of the ovary, generally that nearest to the oviduct, there is a mass of protoplasm, containing nuclei which actively divide; this is the germarium (Fig. 15, A, B, C). As a result of proliferation in the germarium, nucleated masses are thrown off into the cavity of the ovary; each such mass contains four nuclei, and its protoplasm soon becomes divided into four portions, one round each nucleus, so that four cells are produced. In the simpler ovaries, such as that of Leptodora (Fig. 15, A), these sets of four cells are arranged in a linear series within the tube of ovarian epithelium; in other cases, as in Daphnia, the arrangement is more irregular. In the normal development of parthenogenetic eggs, one cell out of each set of four becomes an ovum, the other three feeding it with yolk and then dying. Weismann[32] has shown that the ovum is always formed from the third cell of each set, counting from the germarial end, so that in the ovary of Leptodora drawn in Fig. 15, A, the ova will be formed from the cells marked E1, E2, E3. At certain times, one or two sets of germinal cells fail to produce ova; the epithelial wall of the ovary thickens round these cells, so that they become incompletely separated from the rest in a so-called “nutrient chamber” (Fig. 15, B, N.C). Germ-cells enclosed in a nutrient chamber degenerate and are ultimately devoured by the ovarian epithelium. The significance of these nutrient chambers is unknown.

Fig. 15.—A, Ovary of a parthenogenetic Leptodora hyalina; B, base of another ovary of the same species, showing a so-called “nutrient chamber”; C, ovary of a female Daphnia, showing the formation of a winter-egg. E, E1–E3, Parthenogenetic egg; Ep, ovarian epithelium; G, germarium; N.C, nutrient chamber; O.D, oviduct; W, winter-egg; 1, 2, 4, the other three cells of the same group; II, III, two other groups of germ-cells.
The production of a winter-egg is a more complicated process. The epithelium of the ovarian tube swells up, so that the lumen is nearly obliterated, and several sets of four germ-cells pass from the germarium to lie among the swollen epithelial cells. All these groups of germ-cells, except one, disintegrate and are 46devoured by the ovarian epithelium, one cell of the remaining group enlarging to form a winter-egg, fed during its growth not only by the three cells of its own set but also by the epithelial cells of the ovarian tube, which have devoured the germ-cells of other sets. An ovary never contains more than a single winter-egg at the same time, the number of germ-cells which are devoured during its formation varying in the different species; the Daphnia drawn in Fig. 15, C, has produced three groups of germ-cells, of which two (II, III), will die, while the cell W from the remaining group will develop into an ovum; in Moina, Weismann finds that as many as a dozen cell-groups may be thrown into the ovary before the production of a winter-egg, so that only one out of forty-eight germ-cells survives as an ovum.

Fig. 16.—Sketch of a parthenogenetic Moina rectirostris, × 45, the brood-pouch being emptied and the side of the carapace removed, showing the dome of thickened epithelium on the thorax, by which nutrient material is thrown into the brood-pouch, and the ridge which fits against the carapace in the natural condition so as to close the brood-pouch.

Fig. 17.—Moina rectirostris, ♀, × 40, showing the ephippial thickening of the carapace which precedes the laying of a winter-egg.
The summer-eggs are always carried until they are hatched by the parthenogenetic female which produces them. The brood-pouch is the space between the dorsal wall of the thorax and the carapace. This space is always more or less perfectly closed at the sides by the pressure of the carapace against the body, and behind by vascular processes from the abdominal segments (Figs. 10, 16, etc.). The presence of a large blood-sinus 47beneath the dorsal wall of the thorax and in the middle line of the carapace suggests the possibility that some special nutrient substances may pass from the body of the parent into the brood-chamber, and in some species the thoracic ectoderm is specially modified as a placenta. In Moina (Fig. 16) the dorsal wall of the thorax is produced into a dome, covered by a columnar ectoderm, which contains a dilatation of the dorsal blood-sinus; and in this form it has been shown that the fluid in the brood-pouch contains dissolved proteids. Associated with the apparatus for supplying the brood-pouch with nutriment is a special apparatus for closing it, in the form of a raised ridge, which projects from the back and sides of the thorax and fits into a groove of the carapace.
A somewhat similar nutrient apparatus exists in the Polyphemidae, where the edges of the small carapace are fused with the thorax, so that the brood-pouch is completely closed, and the young can only escape when the parent casts her cuticle. In some genera of this family (e.g. Evadne) the young remain in the parental brood-pouch until they are themselves mature, so that when they are set free they may already bear parthenogenetic embryos in their own brood-pouches.

Fig. 18.—Newly-cast ephippium of Daphnia, containing two winter-eggs.
The winter-eggs are fertilised in the same part of the carapace of the female in which the parthenogenetic eggs develop, but after fertilisation they are thrown off from the body of the mother, either with or without a protective envelope formed from the cuticle of the carapace. The eggs of Sida are surrounded by a thin layer of a sticky substance, and when cast out of the maternal carapace they adhere to foreign objects, such as water-weeds; those of Polyphemus have a thick, gelatinous coat; in Leptodora and Bythotrephes the egg secretes a two-layered chitinous shell. In these forms the cuticle of the 48parent is not used as a protection for the winter-eggs, although it is generally, if not invariably, thrown off when the eggs are laid. In the Lynceidae the cuticle is moulted in such a way that the winter-eggs remain within it, at least for a time; the cuticle is occasionally modified before it is thrown off; thus in Camptocercus macrurus the cuticle of the carapace, in the region of the brood-pouch, becomes thickened and darkly coloured, forming a fairly strong case round the eggs. The modification of the cuticle round the brood-pouch is much more pronounced in the Daphniidae, where it leads to the formation of a saddle-shaped cuticular box, the “ephippium,” in which the winter-eggs are enclosed. The ripening of a winter-egg in the ovary of a Daphnia is accompanied by a great thickening of the cuticle of the carapace (cf. Fig. 18), so that a strong case is formed in the position of the brood-pouch. The winter-eggs are laid between the two valves of this case, and shortly afterwards the parent moults. The eggs are retained within the ephippium, from which the rest of the cuticle breaks away (Fig. 18). After separation, the ephippium, which contains a single egg (Moina rectirostris) or usually two (Daphnia, etc.), either sinks to the bottom, as in Moina, or floats.
The winter-eggs usually go through the early stages of segmentation within a short time after they are laid, but after this a longer or shorter period of quiescence occurs, during which the eggs may be dried or frozen without injury. The sides and floor of a dried-up pond are often crowded with ephippia, containing winter-eggs which develop quickly when replaced in water; and the resting-stage of winter-eggs produced in aquaria can often be materially shortened by drying the ephippia which contain them, though such desiccation does not appear to be necessary for development. Under normal conditions large numbers of winter-eggs remain quiescent through the winter and hatch in the following spring.
The individual developed from a sexually fertilised winter-egg 49is invariably a parthenogenetic female: the characters of the succeeding generations differ in different cases.
In a few forms, of which Moina is the best known, the parthenogenetic female, produced from a winter-egg, may give rise to males, to sexual females, and to parthenogenetic females, so that the cycle of forms which intervene between one winter-egg and the next is short. A sexual female produces one or two winter-eggs, and if these are fertilised they are enclosed in an ephippium and cast off; if, however, the eggs when ripe are not fertilised, they atrophy, and the female produces parthenogenetic eggs, being thenceforward incapable of forming sexual “winter” eggs. An accidental absence of males may thus lead to the occurrence of parthenogenesis in the whole of the second generation. The regular production of sexual individuals in the second generation from the winter-egg appears to depend on a variety of circumstances not yet understood. Mr. G. H. Grosvenor tells me that Moina from the neighbourhood of Oxford may give rise to several successive generations of parthenogenetic individuals, when grown in small aquaria.
In the greater number of Daphniidae, the parthenogenetic female, produced from a winter-egg, gives rise only to parthenogenetic forms, and it is not until after half a dozen parthenogenetic generations have been produced that a few sexual forms appear, mixed with the others. Such sexual forms are fairly common in April or May in this country; they produce “winter” eggs and then die, the generations which succeed them through the summer being entirely parthenogenetic. In late autumn sexual individuals are again produced, giving rise to a plentiful crop of winter-eggs, but many parthenogenetic females are still found, and some of these appear to live and to reproduce through the winter.
In Sida, in the Polyphemidae and Leptodoridae, and in most of the Lynceidae, sexual individuals are produced only once in every year, while in a few forms which inhabit great lakes the sexual condition occurs so rarely that it is still unknown.
Weismann[33] has pointed out that the sexual forms, with their property of producing eggs which can endure desiccation, recur most frequently in species such as Moina, which inhabit small pools liable to be dried up at frequent intervals, while the 50species which produce sexual forms only once a year are all inhabitants either of great lakes which are never dry, or of the sea. Many suggestions have been made as to the environmental stimulus which induces the production of sexual individuals, but nothing is definitely known upon the subject.
We have said that even in those generations which contain sexual males and females there are always some parthenogenetic individuals; there is therefore nothing in the behaviour of Daphniidae, either under natural conditions or when observed in aquaria, to suggest that there is any natural or necessary limit to the number of generations which may be parthenogenetically produced.
The parthenogenetic Daphniidae are extremely sensitive to changes in their surroundings; small variations in the character and amount of substances dissolved in the water are often followed by changes in the length of the posterior spine, in the shape and size of crests on the head, and in other characters affecting the appearance of the creatures, so that the determination of species is often a matter of great difficulty. It is remarkable that the green light which has passed through the leaves of water-plants appears to have a prejudicial effect upon some species. Warren has shown that Daphnia magna reproduces more slowly when exposed to green light, and that individuals grown in this way are more readily susceptible to injury from the presence of small quantities of salt (sodium chloride) in the water than individuals which have been exposed to white light.
The majority of the Cladocera belong to the floating fauna of the fresh waters and seas; a few are littoral in their habits, clinging to water-weeds near the shore, a very few live near the bottom at considerable depths, but the majority belong to that floating fauna to which Haeckel gave the name of “plankton.” The Crustacea are an important element in the plankton, whether in fresh waters or in the sea, the two great groups which contribute most largely to it being the Cladocera and the Copepoda. For this reason it will be more convenient to discuss the habits and distribution of individual Cladocera and Copepoda together in a chapter specially devoted to the characters of pelagic faunas (cf. Chap. VII.). We will only add to the present chapter a table of the families with a diagnosis of the British genera.
Summary of Characters of the British Genera.[34]
Tribe I. Calyptomera, Sars.—The post-cephalic portion of the body enveloped in a free fold or carapace.
A. Six pairs of thoracic feet, the first pair not prehensile (Ctenopoda).
Fam. 1. Sididae: second antennae biramous in both sexes. Sida, Straus (Fig. 11): second antenna with three joints in the dorsal ramus, two in the ventral; the rostrum large, the teeth on the telson many. Latona, Straus: second antenna with two joints in the dorsal ramus, three in the ventral, the proximal joint of the dorsal ramus provided with a setose appendage. Daphnella, Baird: second antenna with the joints as in Latona, but with no setose appendage.
Fam. 2. Holopediidae: second antennae not biramous in the female; a rudimentary second ramus in the male. Holopedium, Zaddach.
B. Four to five or six pairs of thoracic feet, the anterior pair prehensile (Anomopoda).
A. Ventral ramus of second antenna with three joints, the dorsal ramus with four.
Fam. 3. Daphniidae: five pairs of thoracic feet, with a gap between the fourth and fifth pairs. The stomach with two forwardly-directed diverticula.

Fig. 19.—Daphnia obtusa, male, × about 50. Oxford. A.1, First antenna; Th.1, first thoracic appendage.
i. First antennae of female short.
α A median dorsal spine on posterior margin of carapace. Daphnia, O. F. Müller (Fig. 19): first antennae of female not mobile. The head separated from the thorax only by a slight constriction or not at all. Cuticle with a quadrate rhomboid pattern. Ceriodaphnia, Dana: first antennae of 52female mobile. The head separated by a deep depression from the thorax. Cuticle with a polygonal pattern.
β A pair of ventral spines on posterior margin of carapace. Scapholeberis, Schoedler (Fig. 20).

Fig. 20.—Scapholeberis mucronata, female, × 25. Oxford.
γ No spine on posterior margin of carapace. Simocephalus, Schoedler (Fig. 10, p. 39): the cuticle with a pattern of parallel branching ridges.

Fig. 21.—Moina rectirostris, female, × 24. Oxford.
ii. First antennae of female long, mobile. Moina, Baird (Figs. 16, 17, 21): median eye absent. Posterior margin of carapace without a spine.

Fig. 22.—Bosmina sp., female, × about 80. Lake Constance.

Fig. 23.—Acroperus leucocephalus, × about 35. Oxford.
53Fam. 4. Bosminidae: feet equidistant, five or six pairs; the first antennae of the female immobile, with sense-hairs arranged in rings, not forming an apical tuft. The intestine uncoiled; no caeca. Bosmina, Baird (Fig. 22).
Fam. 5. Lyncodaphniidae: four, five, or six pairs of equidistant thoracic limbs; the first two pairs prehensile. First antennae of female mobile, with apical sense-hairs. Intestine coiled or straight.
i. Four pairs of thoracic limbs. Lathonura, Lilljeborg.
ii. Five pairs of thoracic limbs.
a. The four-jointed ramus of the second antenna with four swimming hairs. Macrothrix, Baird: the first antennae of the female flattened, curved. The intestine simple, straight. Streblocerus, Sars: first antennae of the female very little flattened, curved backwards and outwards. The intestine coiled, the stomach with two forwardly-directed caeca.
b. The four-jointed ramus of the second antenna with only three swimming hairs. Drepanothrix, Sars.
iii. Six pairs of thoracic limbs; the labrum provided with an appendage. Acantholeberis, Lilljeborg: appendage of labrum long, pointed, and setose. Intestine without caecum. Ilyocryptus, Sars: appendage of the labrum short, truncated. Intestine with a caecum.
B. Both rami of second antenna three-jointed.
Fam. 6. Lynceidae[35]: five or six equidistant pairs of thoracic feet. Intestine coiled.
i. Six pairs of thoracic limbs. Head and thorax separated by a deep depression. Intestine with one caecum, stomach with two. Female carries many summer-eggs. Eurycercus, Baird.
ii. Five pairs of thoracic limbs. Head and thorax separated by a slight groove or not at all. Anterior digestive caeca absent. Female carries only one or two summer-eggs.
A. Body elongate, oval.
a. Head carinate, the eye far from the anterior cephalic margin. Camptocercus, Baird: body laterally compressed. Second antennae with seven swimming hairs. Telson more than half as long as the shell. Acroperus, Baird (Fig. 23): body compressed. Second antennae with eight swimming hairs, of which one is very small. Telson less than half as long as the shell.
b. Head not carinate, the eye near the anterior cephalic margin. Alonopsis, Sars: terminal claws of telson with three accessory teeth. Alona, Baird: terminal claws of telson with one accessory tooth (includes sub-genera Leydigia, Alona, Harporhynchus, Graptoleberis). Peracantha, Baird (Fig. 14): terminal 54claws of telson with two accessory teeth (includes sub-genera Alonella, Pleuroxus, Peracantha).
B. Body small, spheroidal; the head depressed. Chydorus, Leach: compound eye present. Monopsilus, Sars: compound eye absent.
Tribe II. Gymnomera, Sars.—The carapace forms a closed brood-pouch, which does not cover the body; all the thoracic limbs prehensile.
Fam. 7. Polyphemidae: four pairs of thoracic limbs, provided with a gnathobase.
Fresh-water genera.—Polyphemus, Müller, with no rudimentary exites on first three thoracic limbs. Bythotrephes, Leydig (Fig. 13), with no trace of processes on the outer sides of the limbs.
Marine genera.—Evadne, Lovén, the head not separated by a constriction from the thorax. Podon, Lovén, with deep cervical constriction.

Fig. 24.—Leptodora hyalina, × 6. Lake Bassenthwaite. A.1, First antenna; Car, carapace; I, VI, first and sixth thoracic appendages.
Fam. 8. Leptodoridae: six pairs of thoracic limbs, with no gnathobase. Only genus, Leptodora, Lilljeborg (Fig. 24), from fresh water.
Note.—For extra-European Cladocera consult Daday, “Microskopische Süsswassertiere aus Patagonien und Chili,” Termés Füzetek, xxv., 1902, p. 201; for Paraguay, Bibliotheca Zoologica, Heft 44; for Ceylon, Termés Füzetek, xxi., 1898; and for Australia, Sars, Christiania Vidensk. Forhand. 1885, No. 8, and 1888, No. 7; and Arch. f. Math. og Naturvid. xviii., 1896, No. 3, and xix., 1897, No. 1.—G. W. S.
CHAPTER III
CRUSTACEA (CONTINUED): COPEPODA
Order II. Copepoda.
The Copepods are small Crustacea, composed typically of about sixteen segments, in which the biramous type of limb predominates. They are devoid of a carapace. Development proceeds gradually by the addition posteriorly of segments to a Nauplius larval form. Paired compound eyes are absent, except in Branchiura, the adult retaining the simple eye of the Nauplius.
In a typical Copepod, such as Calanus hyperboreus (Fig. 25), we can distinguish the following segments with their appendages: a cephalothorax, carrying a pair of uniramous first antennae (1st Ant.); a pair of biramous second antennae (2nd Ant.); mandibles (Md.) with biting gnathobases and a palp, and a pair of foliaceous first maxillae (Mx.1). Two pairs of appendages follow, which were looked upon as the two branches of the second maxillae, but it is now certain that they represent two pairs of appendages, which may be called second maxillae (Mx.2) and maxillipedes (Mxp.) respectively. Behind these are five pairs of biramous swimming feet, the first pair (Th.1) attached to the cephalothorax, the succeeding four pairs to four distinct thoracic somites. Behind the thorax is a clearly delimited abdomen composed of five segments, the first of which (Abd.1) carries the genital opening, and the last a caudal furca.
The Copepods exhibit a great variety of structure, and their classification is attended with great difficulties. Claus[36] based his attempt at a natural classification on the character of 56the mouth and its appendages, dividing the free-living and semi-parasitic forms as Gnathostomata from the true parasites or Siphonostomata. This division, although convenient, breaks down in many places, and it is clear that the parasitic mode of life has been acquired more than once in the history of Copepod evolution, while the free-living groups do not constitute a natural assemblage.

Fig. 25.—Calanus hyperboreus, × 30. Abd1, First abdominal segment; 1st Ant, 2nd Ant, 1st and 2nd antennae; Md, mandible; Mx1, Mx2, 1st and 2nd maxillae; Mxp, maxillipede; Th1, 1st thoracic appendage. (After Giesbrecht.)
57Giesbrecht has more recently[37] founded a classification of the free-living pelagic Copepods upon the segmentation of the body and certain secondary sexual characters, and he has hinted[38] that this scheme of classification applies to the semi-parasitic and parasitic forms. Although much detail remains to be worked out and the position of some families is doubtful, Giesbrecht’s scheme is the most satisfactory that has hitherto been suggested, and will be adopted in this chapter.
The peculiarity in structure of the Argulidae, a small group of ectoparasites on fresh water fish, necessitates their separation from the rest of the Copepods (Eucopepoda) as a separate Branch, Branchiura.
BRANCH I. EUCOPEPODA.
Sub-Order 1. Gymnoplea.
The division between the front and hind part of the body falls immediately in front of the genital openings and behind the fifth thoracic feet. The latter in the male are modified into an asymmetrical copulatory organ.
TRIBE I. AMPHASCANDRIA.
The first antennae of the male are symmetrical, with highly-developed sensory hairs.
Fam. Calanidae.—The Calanidae are exclusively marine Crustacea, and form a common feature of the pelagic plankton in all parts of the world. Some species of the genus Calanus often occur in vast shoals, making the sea appear blood-red, and they furnish a most important article of fish food. These swarms appear to consist chiefly of females, the males being taken rarely, and only at certain seasons of the year. Some of the Calanidae are animals of delicate and curious form, owing to the development of plumed iridescent hairs from various parts of their body, which may often exhibit a marked asymmetry, as 58in the species figured, Calocalanus plumulosus (Fig. 26), from the Mediterranean.

Fig. 26.—Calocalanus plumulosus, × 15. (After Giesbrecht.)
Sars makes a curious observation[39] with regard to the distribution of certain Calanidae. He reports that along the whole route of the “Fram,” species such as Calanus hyperboreus and Euchaeta norwegica were taken at the surface, which, in the Norwegian fjords, only occur at depths of over 100 fathoms. He suggests that the Norwegian individuals, instead of migrating northwards as the warmer climate supervened, have sought boreal conditions of temperature by sinking into the deeper waters.
TRIBE II.
HETERARTHRANDRIA.
The first antennae of the male are asymmetrical, one, usually the right, being used as a clasping organ.
The males of the Centropagidae, Candacidae and Pontellidae, besides possessing the asymmetrically modified thoracic limbs of the fifth pair also exhibit a modification of one of the first antennae, which is generally thickened in the middle, and has a peculiar joint in it, or geniculation, which enables it to be flexed and so used as a clasping organ for holding the female.
Fam. 1.—Centropagidae.—These Copepods are very common in the pelagic plankton, and some of the species vie with the 59Calanidae in plumed ornaments, e.g. Augaptilus filigerus, figured by Giesbrecht in his monograph. The use of these ornaments, which are possessed by so many pelagic Copepods, is entirely obscure.[40] Certain of the Centropagidae live in fresh water. Thus Diaptomus is an exclusively fresh-water genus, and forms a most important constituent of lake-plankton; various species of Heterocope occur in the great continental lakes, and certain Eurytemora go up the estuaries of rivers into brackish water.
An excellent work on the fresh-water Copepods of Germany has been written by Schmeil,[41] who gives analytical tables for distinguishing various genera and species. The three fresh-water families are the Centropagidae, Cyclopidae, and Harpacticidae (see p. 62). The Centropagidae may be sharply distinguished from the other fresh-water families by the following characters:—The cephalothorax is distinctly separated from the abdomen; the first antennae are long and composed of 24–25 segments, in the male only a single antenna (generally the right) being geniculated and used as a clasping organ. The fifth pair of limbs are not rudimentary; a heart is present, and only one egg-sac is found in the female. The second antennae are distinctly biramous.
Diaptomus.—The furcal processes are short, at most three times as long as broad; endopodite of the first swimming appendage 2–jointed, endopodites of succeeding legs 3–jointed.
Heterocope.—The furcal processes are short, at most twice as long as broad; endopodites of all swimming legs 1–jointed.
Eurytemora.—The furcal processes are long, at least three and a half times as long as broad; the endopodite of the first pair of legs 1–jointed, those of the other pairs 2–jointed.

Fig. 27.—Dorsal view of Anomalocera pattersoni, ♂, × 20. (After Sars.)
It has been known for a long time that some of the marine Copepods are phosphorescent, and, indeed, owing to their numbers in the plankton, contribute very largely to bring about that liquid illumination which will always excite the admiration of seafarers. In northern seas the chief phosphorescent Copepods belong to Metridia, a genus of the Centropagidae; but in the Bay of Naples Giesbrecht[42] states that the phosphorescent species are the following Centropagids: Pleuromma abdominale and P. gracile, Leuckartia flavicornis and 60Heterochaeta papilligera; Oncaea conifera is also phosphorescent. It is often stated that Sapphirina (p. 69) is phosphorescent, but its wonderful iridescent blue colour is purely due to interference colours, and has nothing to do with phosphorescence. Giesbrecht has observed that the phosphorescence is due to a substance secreted in special skin-glands, which is jerked into the water, and on coming into contact with it emits a phosphorescent glow. This substance can be dried up completely in a desiccated specimen and yet preserve its phosphorescent properties, the essential condition for the actual emission of light being contact with water. Similarly, specimens preserved in glycerine for a long period will phosphoresce when compressed in distilled water. From this last experiment Giesbrecht concludes that the phosphorescence can hardly be due to an oxidation process, but the nature of the chemical reaction remains obscure.
Fam. 2. Candacidae.—This family comprises the single genus Candace, with numerous species distributed in the plankton of all seas. Some species, e.g. C. pectinata, Brady, have a practically world-wide distribution, this species being recorded from the Shetlands and from the Philippines.
Fam. 3. Pontellidae.—This is a larger family also comprising widely distributed species found in the marine plankton. Anomalocera pattersoni (Fig. 27) is one of the commonest elements in the plankton of the North Sea.
Sub-Order 2. Podoplea.
The boundary between the fore and hind part of the body falls in front of the fifth thoracic segment. The appendages of the fifth thoracic pair in the male are never modified as copulatory organs.
TRIBE I. AMPHARTHRANDRIA.
The first antennae in the male differ greatly from those in the female, being often geniculated and acting as prehensile organs.

Fig. 28.—Euterpe acutifrons, ♀, × 70. Abd.1, 1st abdominal segment; Th.5, 5th thoracic segment. (After Giesbrecht.)

Fig. 29.—First antenna of Euterpe acutifrons, ♂. (After Giesbrecht.)
Fams. 1–2. Cyclopidae and Harpacticidae, and other allied families, are purely free-living forms; they are not usually pelagic in habit, but prefer creeping among algae in the littoral zone or on the sea-bottom, or especially in tidal pools. Some genera are, nevertheless, pelagic; e.g. Oithona among Cyclopidae; Setella, Clytemnestra, and Aegisthus among Harpacticidae.
The sketch (Fig. 28) of Euterpe acutifrons ♀, a species widely 62distributed in the Mediterranean and northern seas, exhibits the structure of a typical Harpacticid, while Fig. 29 shows the form of the first antenna in the male.
Several fresh-water representatives of these free-living families occur. The genus Cyclops (Cyclopidae) is exclusively fresh-water, while many Harpacticidae go up into brackish waters: for example on the Norfolk Broads, Mr. Robert Gurney has taken Tachidius brevicornis, Müller, and T. littoralis, Poppe; Ophiocamptus brevipes, Sars; Mesochra lilljeborgi, Boeck; Laophonte littorale, T. and A. Scott; L. mohammed, Blanchard and Richard; and Dactylopus tisboides, Claus.
Schmeil[43] gives the following scheme for identifying the fresh-water Cyclopidae and Harpacticidae (see diagnosis of Centropagidae on p. 59):—
Fam. 1. Cyclopidae.—The cephalothorax is clearly separated from the abdomen. The first antennae of the female when bent back do not stretch beyond the cephalothorax; in the male both of them are clasping organs. The second antennae are without an exopodite. The fifth pair of limbs are rudimentary, there is no heart, and the female carries two egg-sacs.
Cyclops.—Numerous species, split up according to segmentation of rudimentary fifth pair of legs, number of joints in antennae, etc.
Fam. 2. Harpacticidae.—The cephalothorax is not clearly separated from the abdomen. The first antennae are short in both sexes, both being clasping organs in the male. The second antennae have a rudimentary exopodite. The fifth pair of limbs are rudimentary and plate-shaped; a heart is absent, and the egg-sacs of the female may be one or two in number.
1. Ophiocamptus (Moraria).—Body worm-shaped; first antennae of female 7–jointed, rostrum forming a broad plate.
2. Body not worm-shaped; first antennae of female 8–jointed, rostrum short and sharp.
(a) Endopodites of all thoracic limbs 3–jointed. The first antennae in female distinctly bent after the second joint. Nitocra.
(b) Endopodite of at least the fourth limb 2–jointed; first antennae in female not bent. Canthocamptus.
3. Ectinosoma.—Body as in 2, but first antennae are very short, and the maxillipede does not carry a terminal hooked seta as in 1 and 2.
63Fam. 3. Peltiidae.[44]—This is an interesting family, allied to the Harpacticidae, and includes species with flattened bodies somewhat resembling Isopods, and a similar habit of rolling themselves up into balls. No parasitic forms are known, though Sunaristes paguri on the French and Scottish coasts is said to live commensally with hermit-crabs.
We have now enumerated the chief families of free-living Copepods; the rest are either true parasites or else spend a part of their lives as such. A number of the semi-parasitic and parasitic Copepods can be placed in the tribe Ampharthrandria owing to the characters of their antennae; but it must be remembered that many parasitic forms have given up using the antennae as clasping organs; however, the sexual differences in the antennae, and the fact that many of the species which have lost the prehensile antennae in the male have near relations which preserve it, enable us to proceed with some certainty. The adoption of this classification necessitates our separating many families which superficially may seem to resemble one another, e.g. the semi-parasitic families Lichomolgidae and Ascidicolidae, and the Dichelestiidae from the other fish-parasites; it also necessitates our treating the presence of a sucking mouth as of secondary importance. This characteristic must certainly, however, have been acquired more than once in the history of the Copepods, for instance in the Asterocheridae and in the fish-parasites, while it sometimes happens that genera belonging to a typically Siphonostomatous group possess a gnathostome, or biting mouth, e.g. Ratania among the Asterocheridae. Again, it is impossible even if we use the character of the mouth as a criterion to place together all the true parasites on fishes in one natural group, because the Bomolochidae and Chondracanthidae, which are otherwise closely similar to the rest of the fish-parasites, possess no siphon. It seems plain, therefore, that the parasitic habit has been acquired several times separately by diverging stocks of free-swimming Copepods, and that it has resulted in the formation of convergent structures.

Fig. 30.—Haemocera danae, × 40. A, Side view ♀; B, ventral view ♂. Ant.1, 1st antenna; e, eye; ov, ovary; ovd, oviduct; St, stomach; Th.1, 1st thoracic appendage; Th.5, 5th thoracic segment; vd, vas deferens. (After Malaquin.)

Fig. 31.—Free-swimming Nauplius larva of Haemocera danae; Ant.1, Ant.2, 1st and 2nd antennae; e, remains of eye; Md, mandible. (After Malaquin.)
Fam. 4. Monstrillidae.[45]—These are closely related to the Harpacticidae. The members of this curious family are parasitic during larval life and actively free-swimming when adult. There are three genera, Monstrilla, Haemocera, and Thaumaleus. The best known type is Haemocera danae (often described as Monstrilla danae). In the adult state (Fig. 30) there are no mouth-parts; the mouth is exceedingly small and leads into a very small stomach, which ends blindly, while the whole body contains reserve food-material in the form of brown oil-drops. The sole appendages on the head are the first antennae; but on the thorax biramous feet are present by means of which the animal can swim with great rapidity. This anomalous organisation receives an explanation from the remarkable development through which the larva passes. The larva is liberated from the parent as a Nauplius with the structure shown in Fig. 31; it does not possess an alimentary canal. It makes its way to a specimen of the Serpulid worm, Salmacina dysteri, into the epidermis of which it penetrates by movements of the antennae, hanging on all the time by means of the hooks on the mandibles. From the epidermis it passes through the muscles into the coelom of the worm, and thence into the blood-vessels, usually coming to rest in the ventral blood-vessel. 65As the Nauplius migrates, apparently by amoeboid movements of the whole body, it loses all its appendages, the eye degenerates, and the body is reduced to a minute ovoid mass of cells, representing ectoderm and endo-mesoderm, surrounded by a chitinous membrane (Fig. 32, A). Arrived in the ventral blood-vessel it begins to grow, and the first organ formed is a pair of fleshy outgrowths representing the second antennae (Fig. 32, B), which act as a nutrient organ intermediary between host and parasite. The adult organs now begin to be differentiated, as shown in Fig. 32, C, from the undifferentiated cellular elements of the Nauplius, the future adult organism being enclosed in a spiny coat from which it escapes. At this stage it occupies a large part of its host’s body, lying in the distended ventral blood-vessel, and it escapes to the outside world by rupturing the body-wall of the worm, leaving behind it the second antennae, which have performed their function as a kind of placenta. Malaquin, to whom we owe this account, makes the remarkable statement that if two or three Monstrillid Nauplii develop together in the same host they are always males, if only one it may be either male or female. The only parallel to this extraordinary life-history is found in the Rhizocephala (see pp. 96–99).

Fig. 32.—Later stages in the development of Haemocera danae. Abd, Abdomen; Ant.1, Ant.2, 1st and 2nd antennae; ch, chitinous investment; e, eye; Ect, ectoderm; En, endoderm; Mes, mesoderm; Mes & en, mesoderm and endoderm; R, rostrum; St, mouth and stomach; Th, thoracic appendages. (After Malaquin.)

Fig. 33.—Side view of Doropygus pulex, ♀, × 106. Abd.1, 1st abdominal segment; Ant.1, 1st antenna; b.p, brood-pouch; Th.1, 1st thoracic appendage; Th.4, 4th thoracic segment. (After Canu.)
Fam. 5. Ascidicolidae.[46]—Although the members of this family, which live semiparasitically in the branchial sac or the gut of Ascidians, betray their Ampharthrandrian nature by the sexual differences of their first antennae, only two genera, Notodelphys and Agnathaner, possess true prehensile antennae. According as the parasitism is more or less complete, the buccal appendages either retain their masticatory structure or else become reduced to mere organs of fixation. In Notodelphys both sexes can swim actively and retain normal mouth-parts; they live parasitically, or perhaps commensally, in the branchial cavities of Simple or Compound Ascidians, feeding on the particles swept into the respiratory chamber of the host. They leave their host at will in search of a new home, and are frequently taken in the plankton.
Doropygus (Fig. 33), a genus widely distributed in the North Sea and Mediterranean, also inhabiting the branchial sac of Ascidians, is more completely parasitic, and the female cannot swim actively. Forms still more degraded by a parasitic habit are Ascidicola rosea (especially abundant in the stomach of 67Ascidiella scabra at Concarneau), in which the female has lost its segmentation, the mouth-parts and thoracic legs being purely prehensile, and various species of Enterocola, parasitic in the stomach of Compound Ascidians, in which the female is a mere sac incapable of free motion, while the male preserves its swimming powers and a general Cyclops-form (Fig. 34). We have here the first instance of the remarkable parallelism between the degree of parasitism and the degree of sexual dimorphism, a parallelism which holds with great regularity among the Copepoda, and can be also extended to other classes of parasitic animals.

Fig. 34.—Enterocola fulgens. A, Ventral view of ♀, × 35; B, side view of ♂, × 106. Abd.1, 1st abdominal segment; Ant.1, Ant.2, 1st and 2nd antennae; c.m, gland-cells; n, ventral nerve-cord; og, oviducal gland; ov, ovary; po, vagina; Th.1, 1st thoracic appendage; Th.4, Th.5, 4th and 5th thoracic segments. (After Canu.)

Fig. 35.—Asterocheres violaceus, ♀, with egg-sacs, × 57. (After Giesbrecht.)

Fig. 36.—Diagrammatic transverse section through the distal part of the siphon of Rhynchomyzon purpurocinctum (Asterocheridae). Md, mandible. (After Giesbrecht.)
Fam. 6. Asterocheridae.[47]—These forms retain the power of swimming actively, and are very little modified in outward appearance by their parasitic mode of life (Fig. 35), though they 68possess a true siphon in which the styliform mandibles work. The siphon is formed by the upper and lower lips, which are produced into a tube with three longitudinal ridges; in the outer grooves are the mandibles, while the inner groove forms the sucking siphon (see transverse section, Fig. 36). In Ratania, however, there is no siphon. The first antennae possess a great number of joints, and may be geniculated in the male (Cancerilla). The members of this family live as ectoparasites on various species of Echinoderms, Sponges, and Ascidians, but they frequently change their hosts, and it appears that one and the same species may indifferently suck the juices of very various animals, and even of Algae. Cancerilla tubulata, however, appears to live only on the Brittle Starfish, Amphiura squamata.
Fam. 7. Dichelestiidae.—The males and females are similarly parasitic, and the body in both is highly deformed, the segmentation being suppressed and the thoracic limbs being produced into formless fleshy lobes; they are placed among the Ampharthrandria owing to sexual differences in the form of the first antennae. There is a well-developed siphon in which the mandibular stylets work, except in Lamproglena, parasitic on the gills of Cyprinoid fishes; the succeeding mouth-parts are prehensile.
The majority of the species are parasitic on the gills of various fish (Dichelestium on the Sturgeon, Lernanthropus[48] on Labrax lupus, Serranus scriba, etc.), but Steuer[49] has recently described a Dichelestiid (Mytilicola) from the gut of Mytilus galloprovincialis off Trieste. This animal and Lernanthropus are unique among Crustacea through the possession of a completely closed blood-vascular system which contains a red fluid; the older observers believed this fluid to contain haemoglobin, but Steuer, as the result of careful analysis, denies this. The parasite on the gills of the Lobster, Nicothoe astaci, possibly belongs here.
The inclusion of Nicothoe and the Dichelestiidae among the Ampharthrandria rests on a somewhat slender basis; this basis is afforded by the fact that none of the parasitic Isokerandria have more than seven joints in the first antennae, whereas 69Nicothoe and some of the Dichelestiidae[50] have more numerous joints. In most of the Dichelestiidae, however, the number of joints is less than seven and practically equal in the two sexes.
TRIBE II. ISOKERANDRIA.
The first antennae are short, similar in the two sexes, and are never used by the male as clasping organs. This function may be subserved by the second maxillae.
Fams. Oncaeidae, Corycaeidae, Lichomolgidae, Ergasilidae, Bomolochidae, Chondracanthidae, Philichthyidae, Nereicolidae, Hersiliidae, Caligidae, Lernaeidae, Lernaeopodidae, Choniostomatidae.
The families Oncaeidae and Corycaeidae contain pelagic forms of flattened shape and great swimming powers, but the structure of the mouth-parts in the Corycaeidae points to a semi-parasitic habit.
Fam. 1. Oncaeidae.—This family, including the genera Oncaea, Pachysoma, etc., does not possess the elaborate eyes of the next family, nor is the sexual dimorphism so marked.
Fam. 2. Corycaeidae.—These are distinguished from the Oncaeidae, not only by their greater beauty, but also by the possession of very elaborate eyes, which are furnished with two lenses, one at each end of a fairly long tube. The females of Sapphirina are occasionally found in the branchial cavity of Salps, and their alimentary canal never contains solid particles, but is filled with a fluid substance perhaps derived by suction from their prey. S. opalina may occur in large shoals, when the wonderful iridescent blue colour of the males makes the water sparkle as it were with a sort of diurnal phosphorescence. The animal, however, despite the opinion of the older observers, is not truly phosphorescent. It may be that the ornamental nature of some of the males is correlated with the presence of the curious visual organs, which are on the whole better developed in the females than in the males. As in so many pelagic Copepods, the body and limbs may bear plumed setae of great elaboration and beautiful colour, e.g. Copilia vitrea (Fig. 37).
We now pass on to the rest of the parasitic Copepods,[51] which 70probably belong to the tribe Isokerandria, and we meet with the same variety of degrees of parasitism as in the Ampharthrandria, often leading to very similar results.

Fig. 37.—Copilia vitrea (Corycaeidae), ♀, × 20. (After Giesbrecht.)
In the first seven families mentioned below there is no siphon. The Lichomolgidae and Ergasilidae have not much departed from the free-living forms just considered, retaining their segmentation, though in the Ergasilidae the body may be somewhat distorted (Fig. 39). In both families the thoracic swimming feet are of normal constitution.

Fig. 38.—Lichomolgus agilis, × 10. Abd. 1, 1st abdominal segment; cpth, cephalothorax; Th.1, 1st thoracic segment; Th.5, 5th thoracic appendage. (After Canu.)
Fam. 3. Lichomolgidae.[52]—These are semi-parasitic in a number of animals living on the sea-bottom, such as Actinians, 71Echinoderms, Annelids, Molluscs, and Tunicates. Lichomolgus agilis (Fig. 38) occurs in the North Sea, Atlantic, and Mediterranean, on the gills of large species of the Nudibranch, Doris, while L. albeus is found in the peribranchial cavity and cloaca of various Ascidians. Sabelliphilus may infect the gills of Annelids such as Sabella, and is common at Liverpool.
Fam. 4. Ergasilidae.—Thersites (Fig. 39) is parasitic on the gills of various fishes, e.g. T. gasterostei, which is common on Gasterosteus aculeatus on the French and North Sea coasts, and may even be found on specimens of the fish that have run up the River Forth into fresh water. The animal possesses claw-like second antennae by which it clings to its host.

Fig. 39.—Thersites gasterostei. A, ♀, × 10; B, ♂, × 20. Abd. 1 & 2, Fused 1st and 2nd abdominal segments; Ant.1, Ant.2, 1st and 2nd antennae; e.s, egg-sac; Th, thoracic appendages. (After Gerstaecker.)
Similarly characterised by the absence of a siphon are three other families of fish-parasites, the Bomolochidae, Chondracanthidae, and Philichthyidae.
Fam. 5. Bomolochidae.—Bomolochus (Fig. 40), parasitic on the skin of the Sole (Solea) and in the nostrils of Cod (Gadus), is held to be related to the Ergasilidae. The first thoracic limb is remarkably modified. Were it not for the absence of a siphon, it would be hard to separate this family from the Caligidae.
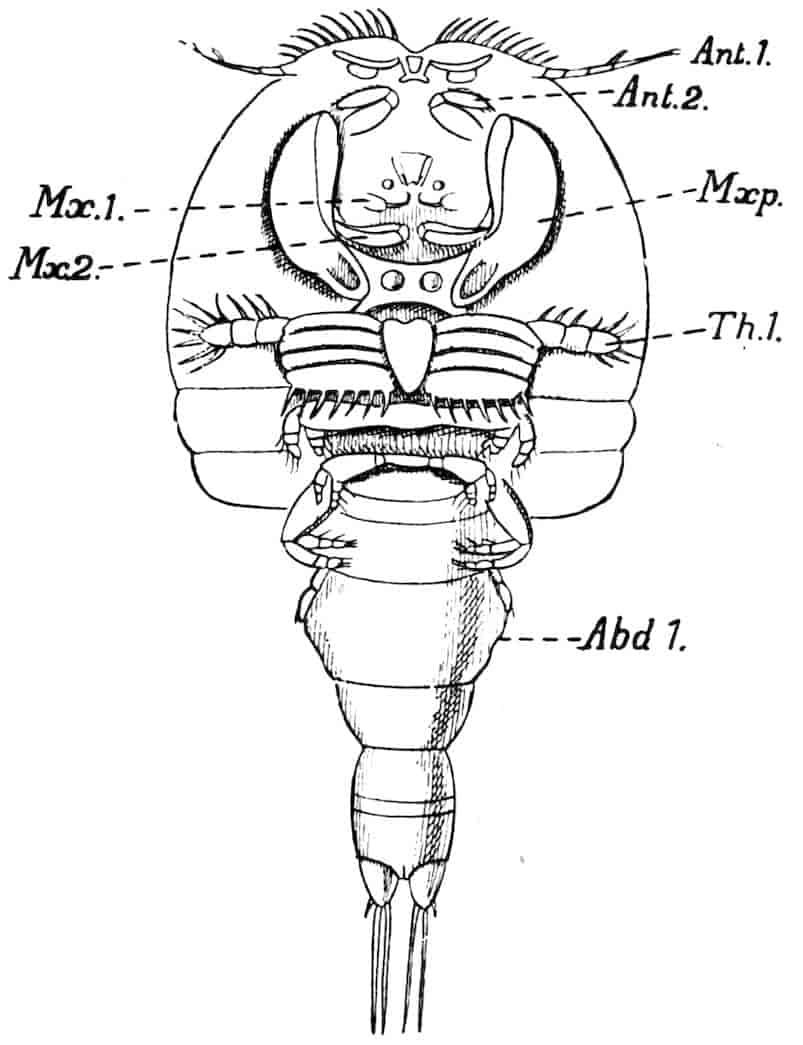
Fig. 40.—Bomolochus, sp. (Bomolochidae), × 8. Abd. 1, 1st abdominal segment; Ant.1, Ant.2, 1st and 2nd antennae; Mx.1, Mx.2, 1st and 2nd maxillae; Mxp, maxillipede; Th.1, 1st thoracic appendage. (After Gerstaecker.)

Fig. 41.—Chondracanthus zei, ♀, × 4.

Fig. 42.—Dwarf male of Lernentoma cornuta (Chondracanthidae), × 10. Ant.1, Ant.2, 1st and 2nd antennae; Th.1, 1st thoracic segment. (After Gerstaecker.)
Fam. 6. Chondracanthidae.—These Copepods infest the gills and even the mouth of various marine fish, such as the Gurnard, Plaice, Skate, Sole, and many others. The sexual dimorphism is very marked, the female being large, indistinctly segmented, and with irregular paired processes protruding from the sides of the body, giving the animal a monstrous form (Fig. 41); while the male (Fig. 42) is very small, has a 73completely segmented thorax, and lives clinging on to the female by its prehensile second antennae—Chondracanthus, Lernentoma.
Fam. 7. Philichthyidae.—These parasites, which are hardly known to occur in British waters,[53] are mucus-feeders and infest the skin of Teleosts, e.g. the Sole; often taking up a position in the lateral line or in a slime canal. They show a similar sexual dimorphism to the foregoing family, the adult female being extraordinarily drawn out into finger-like processes (e.g. Philichthys)[54] or else long, slender, and Nematode-like, with much reduced appendages (Lernaeascus), while the male retains a more normal structure. As in all the foregoing forms there is no siphon.
We now return to two semi-parasitic families, Fam. 8, Nereicolidae, and Fam. 9, Hersiliidae, in which there is certainly no well-developed siphon, but the upper and under lips protrude, forming a hollow between them in which the mouth-parts work. Both families are ectoparasites which frequently leave their hosts, and they retain their segmentation and powers of swimming. Perhaps the best-known form is the Hersiliid, Giardella callianassae, which lives in the adult state in the galleries excavated in the sand by Callianassa subterranea, gaining its nourishment as an ectoparasite on the Decapod. The larvae are pelagic, and are said by Thomson[55] to occur in Liverpool Bay.
List[56] describes Gastrodelphys, a parasite of doubtful position, from the gills of tubicolous worms, such as Myxicola and Sabella, which possesses a perfectly siphonostomatous mouth.
The remaining families to be dealt with are those containing all the fish-parasites which possess a true siphonostome, as well as the siphonostomatous family Choniostomatidae, which is parasitic on other Crustacea. In all these forms the mouth is prolonged into a tube in which the styliform mandibles work.
Fam. 10. Caligidae.—Ectoparasites on fish, lodging most frequently in the gill-chamber. In most of the genera the segmentation and power of swimming are retained in both sexes, the sexual dimorphism not being very well marked, though the males are smaller than the females, and were in some cases originally described as belonging to a special genus Nogagus. 74The females carry two long egg-sacs; the general structure may be made out from the ventral view of Caligus nanus (Fig. 43).
Some of the Caligidae are distinguished by the terga of the thoracic segments being expanded to form large chitinous elytra, e.g. Cecrops, found parasitic on the gills of the Tunny and on the Sun-fish (Orthagoriscus mola). Caligus rapax is parasitic on the skin and in the gills of Sea-Trout, Pollan, etc.; and C. lacustris is common in fresh-water lakes and streams on Pike and Carp.

Fig. 43.—Caligus nanus, × 10. Abd.1, 1st abdominal segment; Ant.1, Ant.2, 1st and 2nd antennae; Mx.1, Mx.2, 1st and 2nd maxillae; Mxp, maxillipede; s, siphon; Th.1, Th.5 1st and 5th thoracic appendages. (After Gerstaecker.)

Fig. 44.—Lernaea branchialis from the Haddock, ♀, × 1. Ceph, cephalothorax; e.s, egg-sacs. (After Scott.)
Fam. 11. Lernaeidae.—These parasites burrow with their heads deep into the skin, or even into the blood-vessels or body-cavity, of various marine fish. The body of the adult female Lernaea is extraordinarily deformed, consisting of a mere shapeless sac with irregular branched processes on the head, and two egg-sacs attached behind (Fig. 44). Pennella sagitta[57] bores so deeply into the flesh of its host, Chironectes marmoratus, that only the egg-sacs and some remarkable branchial processes attached to its abdomen protrude outside the host to the 75exterior. Peroderma cylindricum bores similarly into the flesh of the Sardine, and where it is common, inflicts considerable damage. The males of these curious animals are of more normal structure (Fig. 45). Claus[58] states that fertilisation takes place when both sexes are free-swimming, and of a more or less similar structure, and that subsequently the female becomes fixed to her host and degenerates into the shapeless mass shown in Fig. 44.

Fig. 45.—Lernaea branchialis, ♂, × 10. Ant.1, Ant.2, 1st and 2nd antennae; Br, brain; e, eye; g, stomach; t, testis; vd, vas deferens; ves. sem, vesicula seminalis. (After Claus.)

Fig. 46.—Achtheres percarum. A, ♀, × 4; B, ♂, × 4. Ant.2, 2nd antenna; g, stomach; Mx.2, 2nd maxilla; Mxp, maxillipede; ov, ovary; ovd, oviduct. (After Gerstaecker.)
Fam. 12. Lernaeopodidae.—This family may be illustrated by the common gill-parasite of Perch and Trout, known as Achtheres percarum. The female (Fig. 46), which is much larger than the male, and is not clearly segmented, is attached to the host by means of the maxillipedes, which are fused distally into a pad armed with chitinous hooks. In the male the maxillipedes 76are prehensile, but are not so fused. Besides Achtheres there are other fresh-water forms, e.g. Lernaeopoda salmonea on Salmon, and a number of marine genera. It appears that the larvae fix themselves to their hosts by means of a long glandular thread, which proceeds from the middle of the forehead.[59]

Fig. 47.—Ventral view of Stenochotheres egregius (Choniostomatidae), ♂. A, A′, 1st and 2nd antennae; M, mouth; Mx, 2nd maxilla; T, 1st thoracic leg. (After Hansen.)
Fam. 13. Choniostomatidae.[60]—The members of this family are all parasitic on other Crustacea. The majority live parasitically in the marsupial pouches of female Amphipods, Isopods, Mysidae, and Cumacea, e.g. Sphaeronella and Stenochotheres in the marsupia of Gammarids; but Choniostoma occurs in the branchial cavity of Hippolyte, Homoeoscelis is common in the branchial cavity of Diastylis and Iphinoe, and Aspidoecia on the outside of the body of the Mysid Erythrops. The males and females live together in the same marsupium, but the adult males retain the power of roving about, and do not feed so much as the females, though their mouth-parts are similarly constructed (Fig. 47). Representatives occur all over the world, but the majority of species known at present are from the North Sea, the most abundant being Stenochotheres egregius, parasitic on the Gammarid Metopa bruzelii, Goës.
The male bears a median glandular thread on the forehead by which it attaches itself to the females or to the host. Hansen considers that the family is most closely allied to the Lernaeopodidae.

Fig. 48.—Argulus foliaceus, young ♂, × 15. a1, a2, First and second antennae; ab, abdomen; E, compound eye; l, liver; m, mandibles and first maxillae; mx, second maxilla (the median eye is seen between the two second maxillae); mxp, maxillipede; s.g, shell-gland; sp, spine; t, testis; 1, 4, first and fourth swimming appendages. (After Claus.)
BRANCH II. BRANCHIURA.
Fam. Argulidae.[61]—We have yet to mention this group of fish-parasites, related to the Copepoda, but occupying an isolated position. They are ectoparasites upon various species of fish, Argulus foliaceus being common in the fresh waters of Europe, infesting the branchial chamber or the skin of fresh-water fish, but being frequently taken swimming freely in the water. Both males and females can swim with great agility, and they leave their hosts regularly at the breeding season in spring and autumn; fertilisation is internal, and the female deposits the eggs on stones and other objects. After leaving its host, an Argulus, if it cannot find a fish of the same species, can live on almost any other species, and may even attack Frog tadpoles; while the kinds that infest migratory fish can change with their 78hosts from salt to fresh water, or the reverse. America appears to be the home of the Argulidae.[62]
The structure of an Argulid is exhibited in Fig. 48. In front of the siphon, within which the styliform mandibles and first maxillae work, there is a poison-spine (sp); the appendages which correspond to the second maxillae (mx) are modified into sucking discs, but in the genus Dolops they terminate in normal claws. The next pair of appendages, usually spoken of as maxillipedes (mxp), are clasping organs, and behind follow four pairs of thoracic swimming feet (1–4). The body is foliaceous, and they always apply themselves to their hosts with the long axis pointing forwards and parallel to that of the host, while on various parts of the under surface of the body are spines pointing backward which prevent the parasite being brushed off by the passage of the host through the water. These animals, alone among the Copepoda, possess compound eyes.
A short sketch has now been given of the variations in Copepod organisation, but we cannot leave the subject without pointing out the rich field which still remains for the morphologist, especially in determining the true relationships of the parasitic families.
CHAPTER IV
CRUSTACEA (CONTINUED): CIRRIPEDIA—PHENOMENA OF GROWTH AND SEX—OSTRACODA
Order III. Cirripedia.
The Cirripedes are medium-sized Crustacea, with the body consisting of few segments, and enveloped in a mantle formed as a fold of the external integument, which may be strongly protected by calcified plates. The abdomen is greatly reduced. The larva, after hatching out as a Nauplius, and passing through a Cypris stage, when it resembles an Ostracod, fixes itself to a foreign object by means of the first antennae, and becomes a pupa, which after profound changes gives rise to the adult.
All the Cirripedes, when adult, live either a fixed or parasitic existence, and as so frequently happens with animals of this kind, they have departed widely from the ordinary structure of the class to which they belong. Their anomalous appearance and the mystery surrounding their propagation gave rise, probably, to the old legend that the Barnacles (Lepadidae), which live attached to pieces of floating timber, hatched out into Barnacle geese[63]; and even so late as 1678, in the Royal 80Society’s Transactions, Sir Robert Moray describes what he takes to be little birds enclosed in Barnacle shells, washed ashore on the coast of Scotland: “The little Bill like that of a Goose, the Eyes marked, the Head, Neck, Breast, Wings, Tail, and Feet formed, the Feathers everywhere perfectly shaped and blackish coloured, and the Feet like those of other Water-fowl, to my best remembrance.” Cuvier in his classification of the animal kingdom included them in the Mollusca; and it was not until 1830 that J. V. Thompson described their larval stages, and showed conclusively that they belonged to the Crustacea. Since the work of this naturalist a number of observers have securely founded our knowledge of the group, but we may especially mention the epoch-making works of Darwin,[64] Hoek,[65] and latterly of Gruvel.[66]
The young Cirripede is hatched out from the maternal mantle-cavity as a free-swimming Nauplius, a larval form common to most of the Entomostraca and to some Malacostraca; the Cirripede Nauplius (Fig. 49) is characterised by the presence of well-developed frontal horns, and usually by the long spiny processes which spring from various parts of the body. As an introduction to the study of the group, it will be well to follow the transformations of this larva in Lepas up to the period when it begins its sessile existence. The liberated Nauplii swim freely near the surface of the sea, and remaining in this condition for several days are dispersed widely from their birthplace; they are then transformed by the process of moulting into the second larval stage, known as the Cypris (Fig. 50), from its resemblance to a bivalve Ostracod. The Cypris larva continues to swim about by means of the six pairs of biramous thoracic legs until it finds a suitable place on which to fix; in the case of Lepas fixation usually takes place on loose floating logs; the Cypris fixes itself by means of the first antennae, at the bases of which a large cement-gland secretes an adhesive substance. The biramous swimming legs are cast off, and six pairs of biramous cirri characteristic of the adult take their 81place; at this stage the body has the appearance shown in Fig. 51. The region of the head at the base of the antennae now becomes greatly swollen and elongated to form the peduncle or stalk of the adult; the larval bivalve carapace is cast off and on the external surface of the mantle the calcifications begin which will give rise to the exoskeletal plates of the adult. This region is known as the “capitulum” of the adult, as opposed to the “peduncle.” The young Cirripede is now known as a pupa, and from this stage the adult form is reached by a gradual transition.

Fig. 49.—Nauplius larva of Lepas fascicularis, × 12. A1, A2, 1st and 2nd antennae; B, brain; E, eye; H, fronto-lateral horn; M, mandible; S, stomach. (After Groom.)

Fig. 50.—Cypris stage in the development of Lepas australis, × 15. A, Peduncle; A.M, adductor muscle; C, caecum of oesophagus; C.g, cement-glands; Cr, cirri (thoracic appendages); E, compound eye; E1, simple eye; G, ventral ganglia; I, intestine; M, mouth; M.C, mantle-cavity; O, ovary; S, stomach. (After Hoek.)

Fig. 51.—Pupa of Lepas pectinata, × 8. A, Antenna; C, carina; M, adductor muscle; S, scutum; T, tergum. (After Gruvel.)
The body of the adult Lepas is retracted into the mantle, and lies free in the mantle-cavity, but is continuous anteriorly with the tissues of the peduncle, into which the mantle does not extend. The thorax, with its six pairs of legs, can be protruded from the mantle-cavity through the slit-like opening which separates the two valves of the mantle along the ventral middle line; and when the animal is feeding, the thoracic legs are so protruded, and by their concerted waving action they drive the food-particles in the water along the channel between them, until the particles reach the oral cone, where they are masticated by the mandibles and two pairs of maxillae, and so passed into the alimentary canal. When the animal is disturbed it rapidly retracts its limbs, the valves of the mantle are closed by means of a strong adductor muscle in the head, and the animal is protected from all external influences. In the acorn-barnacles (Operculata), 83which live in great numbers attached to rocks and other objects between tide-marks, the body is constructed on a similar plan, save that there is no stalk, and the body is completely enclosed in a hard calcareous box formed from the mantle, which, when the valves are closed, as they always are during low tide, completely protect the animal inside from desiccation or danger of any kind. Besides the cement-glands situated in the peduncle, we can distinguish the generative organs, consisting of a pair of ovaries and testes, the majority of Cirripedes being hermaphrodite. The testes open at the end of an elongated median penis behind the thoracic limbs, while the ovaries, situated in the peduncle, have paired openings into the mantle-cavity on either side of the head. A pair of maxillary glands or kidneys are present, and the alimentary canal is provided with various digestive glands. Special branchial organs are not present in the Pedunculate Cirripedes, but in the Operculate genera two branchiae are formed from the plications of the internal surface of the mantle. There is no contractile heart, and the circulatory system is poorly developed. The Cirripedes are badly furnished with sensory organs; the remains of a simple Nauplius eye may persist, situated on the upper part of the stomach, but the chief sense-organs are the sensory hairs upon the limbs.

Fig. 52.—A, Dwarf male of Scalpellum vulgare, × 27; B, diagram of Stalked Barnacle. a, Peduncle; al, alimentary canal; b, brain; c, carina; e, remains of Nauplius eye; gl, cement-gland; m, mantle-cavity; o, its opening; ov, ovary; p, penis; s, scutum; t, testis; tm, tergum, seen in A as the shaded body above the reference-line of e and to the right of the carina, on the left of the figure.
The recent Cirripedes fall into six clearly defined Sub-orders.
Sub-Order 1. Pedunculata.
In this division, sometimes combined with the Operculata as Thoracica, owing to the extremely reduced state of the abdomen, the body is borne on a distinct stalk, and the bivalve arrangement of the mantle is clearly retained. The mantle is protected externally by a number of calcareous plates, the arrangement of which is typical of the various genera. It appears that in the most primitive and geologically oldest Cirripedes, the probable ancestors of the Pedunculate and Operculate sub-orders, the arrangement of the plates was somewhat irregular, and they were far more numerous than in the modern forms, so that passing from these older types to modern times we witness a reduction in the number and a greater precision in the arrangement of the skeletal parts.

Fig. 53.—A, Turrilepas wrightianus (Silurian), × 1; B, Archaeolepas redtenbacheri (Jurassic), × 1. C, carina; R, rostrum; S, scutum; T, tergum. (After Zittel.)
One of the most ancient Cirripedes known is Turrilepas, which occurs in the Silurian deposits of England, but it is also known from earlier deposits, while undoubted Cirripedes have been found in the Cambrian of North America. The body of Turrilepas is enclosed in imbricating plates, as shown in Fig. 53, A.
In Archaeolepas of the Upper Jurassic (Lithographic slates of Bavaria) the arrangement of scutes typical of the Lepadidae is foreshadowed, but the whole of the peduncle is protected by rows of plates (Fig. 53, B), as in Turrilepas.
The above-mentioned genera did not survive into the Cretaceous period, their places being taken by the genera Pollicipes and Scalpellum, which first appeared in the Silurian and persist to the present time, the older and more primitive Pollicipes being represented by about half a dozen living species, while the species of Scalpellum are exceedingly numerous.
Fam. 1. Polyaspidae.—This family includes the three genera, Pollicipes, Scalpellum, and Lithotrya.

Fig. 54.—Pollicipes mitella, × 1. (After Darwin.)
Pollicipes is not only very ancient geologically (being found from the Ordovician upward), but it preserves the primitive characteristic 85of numerous skeletal plates, the peduncle being frequently covered with small calcareous pieces, which graduate into the larger more regularly placed scutes on the capitulum (Fig. 54). The species of this genus, many of which are among the largest Cirripedes, are widely distributed in the temperate and tropical seas, living for the most part attached to rocks and often in deep water. P. cornucopia occurs off the English and Scottish coasts.
The members of the genus Scalpellum, which is represented by exceedingly numerous species in the Cretaceous period, also possess a large number of plates on the capitulum, and often on the peduncle as well, but never so many as in Pollicipes. Although the arrangement of the plates varies much in the different species, we may describe a fairly typical case, that of the common Scalpellum vulgare (Fig. 55, B).
The valves of the capitulum are held together by the median dorsal piece called the “carina”; the other unpaired skeletal piece is the “rostrum,” in front, just below the place where the valves gape to allow the protrusion of the limbs. The paired pieces receive the names “scutum,” “tergum,” and “laterals,” and the peduncle is covered with rows of small plates.
The genus Scalpellum is a very large one, and is widely distributed, though at the time at which Darwin wrote only six species were known. The reason for this is to be found in the fact that the great majority of the species live at great depths, so that they remained unknown until the expeditions of the Challenger and other deep-sea expeditions brought them to light. They may affix themselves, generally in considerable numbers together, on branching organisms, such as Corals, Polyzoa, and Hydroids, but often also on empty shells, rocks, and other foreign bodies. The body is colourless or of a pale flesh colour, but a colony of these animals, expanded and drooping in various attitudes from a piece of coral, gives the appearance of some graceful exotic flower.

Fig. 55.—A, Complemental male of Scalpellum peronii, × 20; B, hermaphrodite individual of S. vulgare, × 2. a, Complemental males, in situ; b, rostrum. (A, after Gruvel; B, after Darwin.)
Perhaps the most interesting feature of the genus is the remarkable variation in the sexual constitution of some of the species. The great majority of the Pedunculata and all the Operculata are hermaphrodites, which habitually cross-fertilise one another, and this they are well fitted to do, since they all live gregariously and are provided with a long exsertile penis for transferring the spermatozoa from one to the other. In Pollicipes, however, the individuals of which often live solitarily, it appears that self-fertilisation may occur. In Scalpellum three different kinds of sexual constitution may occur: (1) According to Hoek in S. balanoides, taken by the Challenger, the individuals are ordinary cross-fertilising hermaphrodites. (2) In the great majority of species, including the common S. vulgare, as originally described by Darwin, and since confirmed by Hoek and Gruvel,[67] the individuals are hermaphrodite, but there are present affixed to the adult hermaphrodites, just inside the opening of the valves in a pocket of the mantle, a varying number of exceedingly minute males, called by Darwin “complemental males.” These tiny organisms are really little more 87than bags of spermatozoa, but they possess to varying degrees the ordinary organs of the adult in a reduced condition. The male of S. peronii (Fig. 55, A) retains the shape and skeletal plates of the ordinary form, and differs chiefly in its reduced size; but the more common condition is exhibited by the male of S. vulgare (Fig. 52, A), where the scutes are reduced to vestiges round the mantle-opening, and almost the whole of the body is occupied by the greatly developed generative organs. (3) In a few species, e.g. S. velutinum and S. ornatum, the individuals are purely dioecious, being either females of the ordinary structure resembling the hermaphrodites of the other Lepadidae, or dwarfed males resembling closely the complemental males described above for S. vulgare.

Fig. 56.—Lithotrya dorsalis, x 1. B, Basal calcareous cup; C, carina; R, rostrum; S, scutum; T, tergum. (After Darwin.)
The nature and derivation of these various conditions will be discussed when the parallel cases found in Ibla and among the Rhizocephala have been described.
The remaining genus of the Polyaspidae, also characterised by the presence of numerous skeletal plates on the capitulum, is Lithotrya (Fig. 56), which bores into rocks and shells, and is an inhabitant of the warm and tropical seas.
The peduncle of the full-grown animal is completely imbedded in the rock or shell to which it is attached, and at the basal end of the peduncle is situated a cup composed of large irregular calcified pieces. This cup is, however, not formed until the animal has ceased to burrow. The excavation of the substratum is effected by means of a number of small rasping plates which cover the peduncle, the whole being set in motion by the peduncular muscles.

Fig. 57.—Conchoderma virgata, × 1. C, Carina; S, scutum; T, tergum. (After Darwin.)
Fam. 2. Pentaspidae.—In this family are placed a number of genera, and among them the common Lepas, the species of which possess typically five skeletal plates, viz., a carina and a pair of scuta and of terga, the peduncle being naked. These forms are a later development of Cirripede evolution, and did not come into existence till Tertiary times. Some 88of them, e.g. Oxynaspis, live at considerable depths attached to corals, etc., but large numbers float on the surface of the sea, fixed often on logs and wreckage of various kinds. Dichelaspis is found attached to the shells of large Crustacea.
Conchoderma is an interesting genus, the species of which live affixed to various floating objects, the keels of ships, etc.; the mantle is often brilliantly coloured, as in C. virgata, and the skeletal plates are reduced to the merest vestiges, leaving the greater part of the body fleshy.

Fig. 58.—Ibla cumingii, ♀, × 1. S, Scutum; T, tergum. (After Darwin.)

Fig. 59.—Ibla cumingii, dwarf male, × 32. A, Antennae; B, part of male imbedded in the female, to which the torn membrane M belongs; E, eye; Th, thoracic appendages or cirri. (After Darwin.)
Fam. 3. Tetraspidae.—This family includes the single genus Ibla (Fig. 58), which possesses only four skeletal plates, a pair of terga and of scuta, coloured blue, while the peduncle is covered with brown spines. There are only two very similar species known, I. cumingii, which is found attached to the peduncle of Pollicipes mitella, and I. quadrivalvis, living on masses of the Siphonophore Galeolaria decumbens. These two species are quite different in the partition of the sexes. In I. cumingii the large individuals of normal structure are females, inside the mantle-cavities of which are attached dwarf males of the form shown in Fig. 59.
These organisms have the peduncle buried completely in the substance of the female’s mantle, inside 89which they live; they exhibit a degenerate structure, but still retain two pairs of cirri. The large individuals of I. quadrivalvis, on the other hand, are hermaphrodites, but they harbour within their mantles minute complemental males similar to those of I. cumingii, though they are rather larger.

Fig. 60.—Diagram of the shell of an Operculate Cirripede. a “Ala,” or overlapped portion of a “compartment”; B, basis; C, carina; C.L, carino-lateral; L, lateral; R, rostrum; r, “radius,” or overlapping portion of a compartment; R.L, rostro-lateral. (After Darwin.)
Fam. 4. Anaspidae.—This includes the remaining pedunculate genera, characterised by the fleshy nature of the mantle and peduncle, which are both entirely devoid of calcifications. The species of Alepas live upon Echinoderms and various other animals; Chaetolepas upon Sertularia, and Gymnolepas upon Medusae. Anelasma squalicola is an interesting form, living parasitically upon the Elasmobranch fishes, Selache maxima and Spinax niger in the North Sea. The peduncle is deeply buried in the flesh of the host, so that only a portion of the dark blue capitulum protrudes to the surface. From the whole surface of the peduncle a system of branching processes is given off, which ramify far into the tissues of the fish, and communicate inside the peduncle with the lacunar tissue, which is packed round all the organs of the Cirripede. There can be small doubt that the Anelasma derives its nutriment parasitically through this root-system, since the cirri are mere fleshy lobes unadapted to securing food, and the alimentary canal is always empty. This animal has a suggestive bearing on the Rhizocephala, which, as will be shown, derive their nutriment from a system of roots penetrating the host and growing out from what corresponds morphologically to the peduncle.
Sub-Order 2. Operculata.
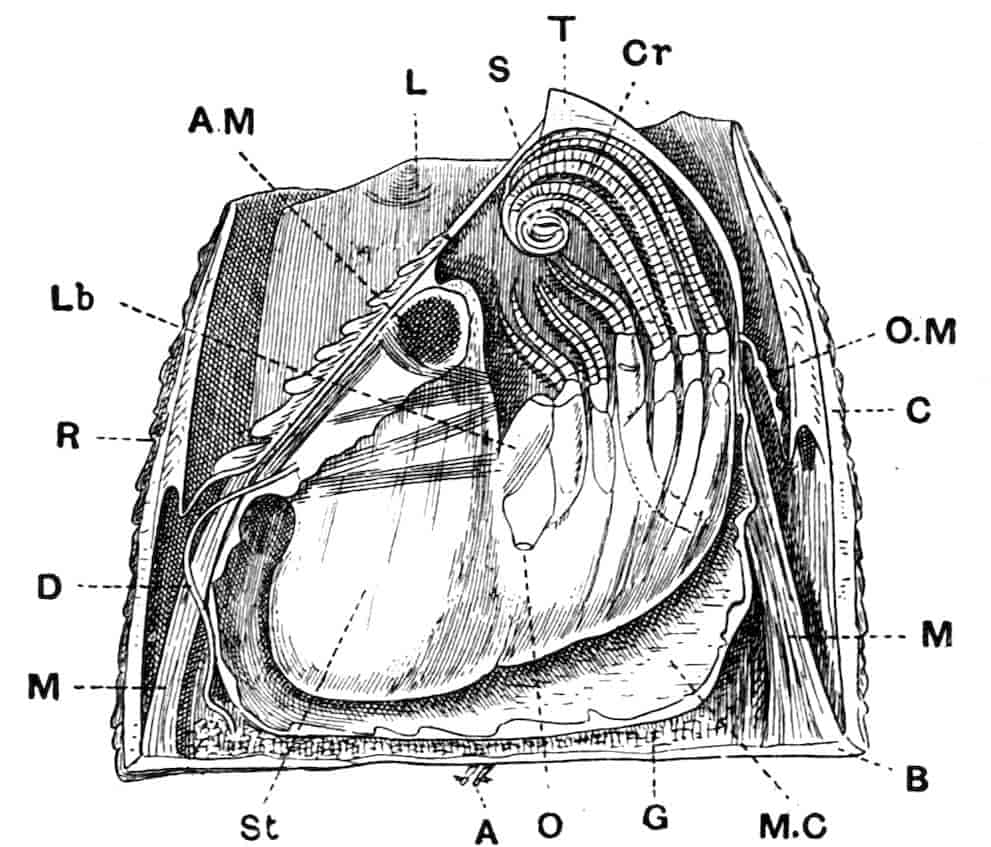
Fig. 61.—Balanus tintinnabulum, with the right half of the shell and of the operculum removed, seen from the right side. A, Antennae, the size of which is exaggerated; A.M, adductor muscle; B, basis; C, carina; Cr, cirri or thoracic appendages; D, oviduct; G, ovary; L, lateral compartment; Lb, labrum or upper lip; M, M, depressor muscles of scutum and tergum; M.C, mantle-cavity; O, orifice of excretory organ; O.M, opercular membrane; R, rostrum; S, scutum; St, region of stomach; T, tergum. (After Darwin.)
The “acorn-barnacles” appear later in geological time than the earlier stalked forms. Verruca and Chthamalus are found in the Chalk, and survive down to the present day, but Balanus does not occur until middle Tertiary times. Representatives of the last-named genus are familiar to every one, 90as the hard sharp objects which cover rocks and piles near high-water mark on every sea-coast. If we examine the hard skeleton of one of these animals, we find that, unlike the Pedunculata, they possess no stalk, the capitulum being fused on to the surface of attachment by a broad basal disc. Typically, there may be considered to be eight skeletal pieces forming the outer ring which invests the soft parts of the animal, an unpaired rostrum and carina, and laterally a pair of rostro-lateral, lateral, and carino-lateral “compartments,” as shown in Figs. 60, 63.
The skeletal ring is roofed over by a pair of terga at the carinal end and a pair of scuta at the rostral end; these four plates make up the operculum by which the animal can shut itself completely up in its shell, or between the valves of which it can protrude its limbs for obtaining food.

Fig. 62.—Diagrammatic section of the growing shell of Balanus porcatus. C, Canals; Ct, cuticle; H, hypodermis (= epidermis); H′, part of shell secreted by the hypodermis; Hl, hypodermal lamina; M, part of shell secreted by the mantle. (After Gruvel.)
The relation of the animal to its shell is shown in Fig. 61. The shell in the Operculata is not merely secreted as a dead structure on the external surface of the epidermis, but represents a living calciferous tissue interpenetrated by living laminae 91(Fig. 62, Hl) derived partly from the external hypodermis and partly from the lining of the mantle. The hard parts of the shell usually also contain spaces and canals (C).

Fig. 63.—Diagrams of shells of Operculata. A, Catophragmus (Octomeridae); B, Balanus, Coronula, etc. (Hexameridae); C, Tetraclita (Tetrameridae). C, carina; C.L, carino-lateral; L, lateral; R, rostrum; R.L, rostro-lateral.
The various forms of Acorn-barnacle may be classified according to the number of pieces that go to make up the skeleton; thus starting with the typical number eight (Fig. 63, A), we find that in various degrees a fusion between neighbouring pieces has taken place in the different families.
Fam. 1. Verrucidae.—The ancient genus Verruca, which is still widely distributed in all seas, and is found fixed upon foreign objects on the sea-bottom at various depths, is interesting on account of the asymmetry of its shell, which bears a different aspect according to which side one regards it from. This asymmetry is brought about by the skeletal pieces (carina, rostrum, and paired terga and scuta) shifting their positions after fixation has taken place.
Fam. 2. Octomeridae.—In this family the eight plates composing the shell are separate and unfused (Fig. 63, A). The majority of the species come from the Southern hemisphere, e.g. the members of the genera Catophragmus and Octomeris, but Pachylasma giganteum occurs in deep water in the Mediterranean, where it has been found fixed upon Millepore corals.
Fam. 3. Hexameridae.—This family includes by far the greater number of the Acorn-barnacles, in which only six plates are present, the laterals having fused with the carino-laterals (Fig. 63, B). The very large genus Balanus belongs here, the common B. tintinnabulum of our coasts being found all over the world, and occurring under a number of inconstant varietal forms. Especial interest attaches to certain other genera, from their habit of living parasitically on soft-bodied animals, whose flesh they penetrate.
Coronula diadema and Tubicinella trachealis live embedded 92in the skin of whales, the shell of the first-named being of a highly complicated structure with hollow triangular compartments into which the mantle is drawn out.
Xenobalanus globicipitis lives attached to various Cetacea, and is remarkable for the rudimentary condition of its skeleton, the six plates of which form a mere disc of attachment from which the greatly elongated naked body rises, resembling one of the naked Stalked Barnacles.
Fam. 4 Tetrameridae.—In this family only four skeletal plates are present (Fig. 63, C). This family is chiefly confined to tropical seas or those of the Southern hemisphere. The chief genera are Tetraclita and Pyrgoma, found in British seas.
Sub-Order 3. Acrothoracica.
Gruvel includes in this sub-order four genera (Alcippe, Cryptophialus, Kochlorine, and Lithoglyptes) the species of which live in cavities excavated in the shells of molluscs or in the hard parts of corals.

Fig. 64.—Alcippe lampas. A, ♀, × about 10, seen from the right side, with part of the right half of the animal removed; B, dwarf male, × about 30. A.M, adductor muscle; An, antenna; C, 1st pair of cirri; Cr, posterior thoracic appendages; E, eye; G, testis; M.C, mantle-cavity; O, ovary; P, penis; T, penultimate thoracic segment; V. vesicula seminalis. (After Darwin.)
Darwin discovered and described Cryptophialus minutus, and placed it in a sub-order Abdominalia, believing that it was 93distinguished from all the foregoing Cirripedes by the presence of a well-developed abdomen. Since the discovery of other allied genera, it has been decided that the abdomen is equally reduced in these forms, and that the terminal appendages do not belong to this region, but to the thorax.
The sexes are separate. The body of the female (Fig. 64, A) is enclosed in a chitinous mantle, armed with teeth by which the excavation is effected, and is attached to the cavity in the host by means of a horny disc. Upon this disc the dwarf males (B) are found.
Alcippe lampas inhabits holes on the inner surface of dead Fusus and Buccinum shells; Cryptophialus minutus the shells of Concholepas peruviana; C. striatus [68] the plates of Chiton; Kochlorine hamata the shells of Haliotis; and Lithoglyptes varians shells and corals from the Indian Ocean.
Sub-Order 4. Ascothoracica.
These are small hermaphrodite animals completely enveloped in a soft mantle, which live attached to and partly buried in various organisms, such as the branching Black Corals (Gerardia). They retain the thoracic appendages in a modified state, and the body is segmented into a number of somites, the last of which probably represents an abdomen.
Laura gerardiae, described by Lacaze Duthiers,[69] is parasitic on the stem of the “Black Coral,” Gerardia (vol. i. p. 406); it has the shape of a broad bean, the body being entirely enclosed in a soft mantle, with the orifice in the position corresponding to the hilum of a bean. The body lying in the mantle is composed of eleven segments, and is curved into an S-shape. Its internal anatomy is entirely on the plan of an ordinary Cirripede.
Petrarca bathyactidis, G. H. Fowler,[70] was found in the mesenteric chambers of the coral Bathyactis, dredged by the Challenger from 4000 metres. The body is nearly spherical, and the mantle-opening forms a long slit on the ventral surface. The mantle is soft, but is furnished on the ventral surface with short spines.
The antennae, which form the organs of fixation, remain 94very much in the state characteristic of the Cypris larvae of other Cirripedes, being furnished with two terminal hooks by which attachment is effected. The thoracic appendages, of which there are the normal number six, are reduced flabellate structures, and the abdomen forms an indefinitely segmented lobe of considerable size.
The animal appears to be in an arrested state of development, and so retains some of the characteristics of the Cypris larvae, but it is very doubtful how far these characters can be considered primitive.
Other forms are Dendrogaster astericola on Echinoderms, and Synagoga mira on the “Black Coral,” Parantipathes larix, at Naples.
Sub-Order 5. Apoda.

Fig. 65.—Proteolepas bivincta, × 26. A, Antennae; a, b, 1st and 2nd abdominal segments; O, ovary; P, penis; T, telson; 1–8, thoracic segments. (After Darwin.)
Darwin described a small hermaphrodite parasite in the mantle chamber of Alepas cornuta from Saint Vincent, West Indies, which he named Proteolepas bivincta.
The body (Fig. 65) is distinctly segmented into eleven somites, the last three of which are supposed to belong to the abdomen; there are no appendages except the antennae by which fixation is effected. The mouth-parts are of normal constitution.
This animal has not been found again since Darwin’s discovery, but Hansen[71] describes a number of peculiar Nauplius larvae taken in the plankton of various regions, which he argues probably belong to members of this group. A wide field of work is offered in attempting to find the adults into which various larvae grow.
Sub-Order 6. Rhizocephala.[72]
These remarkable animals are Cirripedes which have taken to living parasitically on various kinds of Crustacea; the majority infest species of Decapoda, e.g. Peltogaster on Hermit-Crabs, Sacculina on a number of Brachyura, Sylon on Shrimps, Lernaeodiscus on Galathea; but one genus, Duplorbis, has been found in the marsupium of the Isopod Calathura brachiata from Greenland. Most of the species are solitary, but a few, e.g. Peltogaster sulcatus, are social. In the adult state the body consists of two portions: a soft bag-like structure, external to the host, carrying the reproductive, nervous, and muscular organs, and attached to some part of the host’s abdomen by means of a chitinous ring; and a system of branching roots inside the host’s body, which spring from the ring of attachment and supply the external body with nourishment.

Fig. 66.—Nearly median longitudinal section (diagrammatic) of Peltogaster. gn, Brain; m, mantle; mc, mantle-cavity; mes, mesentery; op, mantle-opening; ov, ovary; ovd, oviduct; ring, ring of attachment; t, testis; vd, vas deferens.

Fig. 67.—Diagrammatic median longitudinal section through a normal Cirripede, gn, Brain; op, mantle-opening; ovd, oviduct; vd, vas deferens.
The structure of the external bag-like portion is very simple, and varies only in details, chiefly of symmetry, in the different genera. In Peltogaster, which preserves the simplest symmetrical arrangement of the organs, a diagrammatic section through the long axis of the body (Fig. 66) shows that it consists of a muscular mantle (m) surrounding a visceral mass, and enclosing a mantle-cavity (mc) or brood-pouch, which stretches everywhere between mantle and visceral mass, except along the surface by which the parasite is attached to its host, where a mesentery (mes) is formed. The ring of attachment is situated in the middle of this mesentery; the mantle-cavity, which is completely 96lined externally and internally with chitin, opens anteriorly by means of a circular aperture (op) guarded by a sphincter muscle. The visceral mass is composed chiefly of the two ovaries (ov), which open on either side of the mesentery by means of a pair of oviducts (ovd); the paired testes (t) are small tubes lying posteriorly in the mesentery, and the nervous ganglion (gn) lies in the mesentery between oviducts and mantle-opening. A comparison with the condition of a normal Cirripede (Fig. 67) shows us that the mesenterial surface of the parasite by which it is fixed corresponds to the dorsal surface of an ordinary Pedunculate Cirripede, and that the ring of attachment corresponds with the stalk or peduncle of a Lepas. The root-system passes out through the ring of attachment into the body of the host, and ramifies round the organs of the crab; the roots are covered externally with a thin chitinous investment, and consist of an epithelium and an internal mass of branching cells continuous with the lacunar tissue in the visceral mass.

Fig. 68.—Development of Sacculina neglecta. A, Nauplius stage, × about 70; B, Cypris stage, × about 70. A1, A2, 1st and 2nd antennae of Nauplius; Ab, abdomen; Ant, antenna of Cypris; E, undifferentiated cells; F, frontal horn; G, glands of Cypris; H, tendon of Cypris; M, mandible; T, tentacles.
The developmental history of the Rhizocephala is one of the most remarkable that embryology has hitherto revealed. It has been most accurately followed in the case of Sacculina. The young are hatched out in great numbers from the maternal mantle-cavity as small Nauplii (Fig. 68, A) of a typical Cirripede nature, but without any alimentary canal. They swim near the surface of the sea, and become transformed into Cypris larvae of a typical character (Fig. 68, B). The Cypris larva, after a certain period of free existence, seeks out a crab and fixes itself by means of the hooks on its antennae to a hair on any part of the crab’s body. Various races of Sacculina are known which infest about fifty different species of crabs in various seas; the best known are S. carcini parasitic on Carcinus maenas at Plymouth and Roscoff, and S. neglecta on Inachus mauritanicus at Naples. The antenna, by which the Cypris is fixed, penetrates the base of the hair; the appendages are thrown away, and a small mass of undifferentiated cells is passed down the antenna into the body-cavity of the crab. Arrived in the body-cavity it appears that this small mass of cells is carried about in the blood-stream until it reaches the spaces round the intestine in the thorax. Here it becomes applied to the intestine, usually at its upper part, immediately beneath the stomach of the crab (Fig. 69), and from this point it proceeds to throw out roots in all directions, and as it grows to extend its main bulk, called the central tumour (c.t), towards the lower part of the intestine. As the posterior border of the central tumour grows down towards the hind-gut, the future organs of the adult Sacculina become differentiated in its substance; the mantle-cavity being excavated and surrounding the rudiment of the visceral mass, while as the central tumour grows downwards it leaves behind it an ever extending system of roots. When the central tumour in process of differentiation has reached the unpaired diverticulum 98of the crab’s intestine, at the junction between thorax and abdomen, all the adult organs are laid down in miniature, and the whole structure is surrounded by an additional sac formed by invagination known as the perivisceral space (Fig. 70). The young “Sacculina interna” remains in this position for some time, and being applied to the ventral abdominal tissues of the crab just at the point where thorax and abdomen join, or a little below it, it causes the crab’s epithelium to degenerate, so that when the crab moults, a little hole is left in this region of the same size as the body of the Sacculina, owing to the failure of the epithelium to form chitin here; and thus the little parasite is pushed through this hole and comes to the exterior as the adolescent “Sacculina externa.” From this point onwards the crab, being inhibited in its growth through the action of the parasite, never moults again; so that the Sacculina occupies a safe position protruding from the crab’s abdomen, which laps over it and protects it. The remarkable features of this development are, firstly, the difficulty of understanding how the developing embryo is directed in its complicated wanderings so as always to reach the same spot where it is destined to come to the exterior; and, secondly, the loss after the Cypris stage of all the organs and the resumption of an embryonic undifferentiated state from which the adult is newly evolved. A certain parallel to this history is found in that of the Monstrillidae, described on pp. 64–66.

Fig. 69.—The mid-gut of Inachus mauritanicus with a young Sacculina overlying it, × 2. c.t, “Central tumour” of the parasite; d.i, d.s, inferior and superior diverticula of alimentary canal of host; n, “nucleus,” or body-rudiment of Sacculina; r, its roots; x, definitive position of the parasite.

Fig. 70.—Later stage in the development of the “Sacculina interna,” × 2. b, Body of Sacculina; c.t, “central tumour”; d.i, d.s, inferior and superior diverticula of alimentary canal of host; o, opening of perivisceral cavity of Sacculina; r, its roots.

Fig. 71.—Fourteen Cypris larvae fixed round the mantle-opening (o) of a young Sacculina externa, × 20.
The Rhizocephala are hermaphrodite with the possible exception of Sylon, which appears to be female and perhaps parthenogenetic, no male having been seen; but unlike most other hermaphrodite Cirripedes, they reproduce by a continual round of self-fertilisation. This is the more remarkable in that the vestiges of what appears to be a male sex are still found in Sacculina and Peltogaster; certain of the Cypris larvae in these genera, instead of fixing on and inoculating other crabs, become attached round the mantle-openings of young parasites of the same species as themselves, which have recently attained to the exterior of their hosts (Fig. 71). These larvae, which remind us of the complemental males in Scalpellum, etc., never produce spermatozoa, but rapidly degenerate where they are fixed, and appear never to play any rôle in the reproduction of their species. The nature of this remarkable phenomenon, together with the sexual condition of the Cirripedes in general, will be discussed in the next section.
Much remains to be elucidated in the life-histories of these curious animals, and it seems probable that intermediate stages may exist, showing us how the extreme discontinuity of development has been reached. Suggestive in this respect is the newly discovered parasite of the Isopod, Calathura, which the author has named Duplorbis calathurae.[73] This animal does not appear 100to possess a root-system, but is attached to its host by a tube which passes right through the mesentery and opens into the mantle-cavity of the parasite. It may be suggested that this tube corresponds to the stalk of the normal Cirripede, but its exact mode of formation would certainly throw much light on the question of Rhizocephalan development.
Phenomena of Growth and Sex in the Crustacea.
In the foregoing account of the Cirripedia we have met with certain peculiar sexual relations in which closely allied species exhibit marked differences in regard to the distribution of the qualities of sex among their individuals; we have seen that the majority of species are hermaphrodite, unlike most Crustacea which, with the other exception of the parasitic Isopoda, are normally dioecious; and that in some species complemental males exist side by side with the hermaphrodites, while, in yet others, the individuals are either females or dwarf males.
Before examining the causes of these conditions, it will be opportune to consider a number of facts which throw light on the question of sex and hermaphroditism in general. We may then return to the discussion of the hermaphroditism found in particular in the Cirripedia and Isopoda.
Parasitic Castration.—Giard[74] was the first to observe that a number of parasites exert a remarkable influence on the sexual characters of their host, such that the generative glands become reduced, or may completely degenerate, while the secondary sexual characters become materially altered. This was proved to occur in the most widely different hosts, affected by the most widely different parasites (e.g. Crustacea, Insecta, Worms). Moreover, it was apparent that the affection does not consist in the parasite merely destroying the generative organs, with which it often does not come into contact, but rather in the general disturbance of the metabolism set up by its presence.
The most completely studied cases of parasitic castration are those of the Rhizocephalous Sacculina neglecta, parasitic on the spider-crab, Inachus mauritanicus,[75] and of Peltogaster curvatus 101on the Hermit-crab, Eupagurus excavatus, var. meticulosa.[76] The ordinary males of I. mauritanicus have the appearance shown in Fig. 72, A. The abdomen is small and bears a pair of copulatory styles, while the chelipedes are long and swollen. In the female (B) the abdomen is much larger and trough-shaped, and carries four pairs of ovigerous appendages; the chelae are small and narrow.

Fig. 72.—Illustrating the effect of parasitic Sacculina neglecta on Inachus mauritanicus, nat. size. A, Normal male; Inachus; B, normal female; C, male infested by Sacculina (final stage); D, abdomen of infested female; E, infested male in an early stage of its modification.
Now it is found that in about 70 per cent of males infected with Sacculina the body takes on to varying degrees the female characters, the abdomen becoming broad as in the female, with a tendency to develop the ovigerous appendages, while the chelae become reduced (Fig. 72, C). This assumption of the female characteristics by the male under the influence of the parasite may be so perfect that the abdomen and chelae become typically female in dimensions, while the abdomen develops not only the 102copulatory styles typical of the male, but also the four pairs of ovigerous appendages typical of the female. The parasitised females, on the other hand, though they may show a degenerate condition of the ovigerous appendages (Fig. 72, D), never develop a single positively male characteristic. On dissecting crabs of these various categories it is found that the generative organs are in varying conditions of degeneration and disintegration.
The most remarkable fact in this history is the subsequent behaviour of males which have assumed perfect female external characters, if the Sacculina drops off and the crabs recover from the disease. It is found that under these circumstances these males may regenerate from the remains of their gonads a perfect hermaphrodite gland, capable of producing mature ova and spermatozoa. The females appear quite incapable, on the other hand, of producing the male primary elements of sex on recovery, any more than they can produce the secondary. Exactly analogous facts have been observed in the case of the hermit-crabs parasitised by Peltogaster, but here the affected males produce small ova in their testes before the parasite is got rid of. Here, too, the females seem incapable of assuming male characters under the influence of the parasite.
To summarise shortly the conclusions to be deduced from these facts—certain animals react to the presence of parasites by altering their sexual condition. This alteration consists in the female sex in an arrest of reproductive activity, in the male sex in the arrest of reproductive activity coupled with the assumption of all the external characters proper to the female. But in these males it is not merely the external characters that have been altered; their capacity for subsequently developing hermaphrodite glands shows that their whole organisation has been converted towards the female state. That this alteration consists in a reorganisation of the metabolic activities of the body is clearly suggested, and in the succeeding paragraph we furnish some further evidence in support of this view.

Fig. 73.—Inachus mauritanicus, × 1. A, Low male; B, middle male; C, high male; the great chela of the right side is the only appendage represented.
Partial and Temporary Hermaphroditism. High and Low Dimorphism.
The reproductive phases of animals are frequently rhythmic, periods of growth alternating with periods of reproduction. This is well exemplified in the case of the ordinary males of Inachus mauritanicus, of some other Oxyrhynchous crabs, and of the Crayfish Cambarus.[77] During the breeding season the males of I. mauritanicus fall into three chief categories: Small males with swollen chelae (Fig. 73, A), middle-sized males with flattened chelae (B), and large males with enormously swollen chelae (C). On dissecting specimens of the first and third categories it is found that the testes occupy a large part of the thoracic cavity and are full of spermatozoa, while in the middle-sized males with female-like chelae the testes appear shrivelled and contain few spermatozoa. These non-breeding crabs are, in fact, undergoing a period of active growth and sexual suppression before attaining the final state of development exhibited by the large breeding males. This phenomenon is obviously parallel to the “high and low dimorphism”[78] so common in Lamellicorn beetles, where the males of many species are divided into two chief categories, viz. “low males” of small size in which the secondary sexual characters are poorly developed, and “high males” of large size in which these characters are proportionately 104much more highly developed than in the low males. The only difference between the two cases is that whereas in the beetles growth ceases on the attainment of maturity in the low degree, in the Crustacea the low male passes through a period of growth and sexual suppression to reach the high degree of development.
The condition of the middle-sized males may be looked upon as one of partial hermaphroditism, indications of the female state being found in the flattened chelae and in the reduced state of the testes. This interpretation is greatly strengthened by the state of affairs observed in the life-history of the male Sand-hoppers, Amphipods of the genus Orchestia.[79] In the young males of several species of this genus, at the time of year when they are not actively breeding, small ova are developed in the upper part of the testes of more than half of the male individuals, these ova being broken down and reabsorbed as the breeding season reaches its height. Nor is this phenomenon confined to this genus; in the males of a number of widely different Crustacea these small ova are found in the testes at certain periods of the life-history (e.g. Astacus[80]), when the animal is not breeding.
The foregoing facts indicate unmistakably that the males of a number of Crustacea under certain metabolic conditions, i.e. when a stage of active growth as opposed to a stage of reproductive activity is initiated, alter their sexual constitution in such a way that the latent female characteristics are developed, and the organism appears as a partial hermaphrodite. In the preceding paragraph we saw that the males of a number of animals, especially Crustacea, react to the metabolic disturbance set up by the presence of a parasite in exactly the same way, i.e. by developing into partial or total hermaphrodites. From these two converging bodies of facts we may conclude, firstly, that sex and metabolism are two closely connected phenomena; and, secondly, that the male sex is especially liable to assume hermaphrodite characters whenever its metabolic requirements are conservative, assimilatory, or in a preponderating degree anabolic, as when a phase of active growth is initiated, or the drain on the system, due to the presence of a parasite, is to be made good.
Normal Hermaphroditism in Cirripedia and Isopoda Epicarida.
The above-mentioned groups contain the only normally hermaphrodite Crustacea, and since they are in most respects highly specialised, we may be certain that they have been secondarily derived from dioecious ancestors. They both lead a sessile or parasitic life, and it is noteworthy that this habit is often associated with hermaphroditism, e.g. in Tunicates. A sessile or parasitic mode of life is one in which the metabolic functions are vegetative and assimilatory rather than actively kinetic or metabolic. It is in this state that we have seen the males of a number of Crustacea taking on a temporary or partial hermaphroditism. We may, therefore, inquire, whether in these cases of normal hermaphroditism there is any evidence to show that here too the hermaphroditism has been acquired by the male sex as a response to the change in the metabolic conditions. In the parasitic Isopoda Epicarida (see pp. 129–136) the hermaphroditism is of a very simple kind; all the individuals are at first males, whose function it is to fix on and fertilise the adult parasites. These subsequently develop into females which are in their turn cross-fertilised by the young larvae derived from a previous generation. All the individuals being alike, it seems probable that they have been derived from one sex, and the general nature of hermaphroditism deduced above may lead us to suppose that that sex was originally male, the female having been suppressed. In certain Cirripedia, e.g. most species of Scalpellum, there exist, besides the hermaphrodite individuals, complemental males, so that here a superficial conclusion might be drawn that the hermaphrodites represent the female sex. But if we can suggest that the complemental males are in reality similar in derivation to the hermaphrodite individuals, we shall be in a position to claim that the hermaphrodite Cirripedes are similar to the Isopoda Epicarida, and have probably also been derived from the male sex. There is decided evidence pointing to this conclusion. In the first place, the complemental males of at least one species of Scalpellum, S. peronii, do show an incipient hermaphroditism[81] in the presence 106of small ova in their generative glands, which, however, never come to maturity.
The condition of the degenerate males in the Rhizocephala may also be interpreted in the same manner. These never pass beyond the Cypris stage of development, in which they resemble in detail the Cypris larvae of the ordinary hermaphrodite individuals, and they are quite useless in the propagation of their species.
It is more reasonable to suppose that these Cypris larvae, which fix on the mantle-openings of adult parasites, are in reality identical with the ordinary Cypris which infest crabs and develop into the hermaphrodites, than that they represent a whole male sex doomed beforehand to uselessness and degeneration. If we suppose that the Cirripedes have passed through a state of protandric hermaphroditism similar to that of the Isopoda Epicarida, it is plain that all the larvae must have originally possessed the instinct of first fixing on the adult parasites, and we may suppose that this instinct has been retained in the Rhizocephala, but is now only actually fulfilled by a certain proportion of the larvae, which, under existing circumstances, are useless and fail to develop further; while the rest of the larvae, not finding an adult parasite to fix upon, go straight on to infect their hosts and develop into the adult hermaphrodites.
The same explanation would apply to the complemental males in Scalpellum, etc., these individuals being also potential hermaphrodites, which are arrested in development, though not so completely as in the Rhizocephala, owing to the position they have taken up.
This theory throws light on another dark feature of Cirripede life-history, namely, the gregarious instinct. The associations of Cirripedes are not formed by a number of Cypris larvae fixing together on the same spot, but rather by the Cypris larvae seeking out adolescent individuals of their own species and fixing on or near them. Now, if we suppose that the Cirripedes have passed through a condition of protandric hermaphroditism similar to that of the Isopoda Epicarida, it is clear that a slight modification of the sexual instinct of the larvae would lead to the gregarious habit, while its retention in some individuals in its original form accounts for their finding their way to the 107mantles of adult individuals and developing into the so-called complemental males.
Certain Cirripedes, viz. certain species of Scalpellum and Ibla and all the Acrothoracica, are dioecious. It is impossible to decide at present whether these species retain the primitive dioecious condition of the ancestral Cirripedes, or whether they too have been derived from an hermaphrodite state, but in the present state of knowledge they hardly affect the validity of the theory that has been proposed to account for the nature of the complemental males and the hermaphrodite individuals.
Order IV. Ostracoda.
The Ostracoda are small Crustacea, the body consisting of very few—about eight—segments, and being completely enclosed in a carapace, which has the form of a bivalve shell. Development is direct, without a Nauplius stage.
The Ostracoda[82] are marine and fresh-water animals that can be divided into several families, differing slightly in habits and in structures correlated with those habits.

Fig. 74.—Candona reptans. A, Natural size; B, X 15. a, 1st antennae; b, 2nd antennae; c, walking legs. (After Baird.)
The Cypridae and Cytheridae include all the fresh-water and a vast majority of marine genera, adapted for a sluggish life among water-plants, though some can swim with considerable activity. The common Cypris and Candona of our ponds and streams are familiar instances. The movements of these animals are effected by means of the two pairs of uniramous pediform antennae which move together and in a vertical straight line. In the Cypridae (Fig. 74) there are, besides the mandibles, two pairs of maxillae, a pair of walking legs, and, lastly, a pair of appendages, which are doubled up into the carapace, and are used for cleaning purposes. In the marine Cytheridae there is only one maxilla, the last three appendages 108being pediform and used in walking. The telson in the Cytheridae is rudimentary, but is well developed in the Cypridae. The heart is altogether absent.
In many of the fresh-water forms, e.g. common species of Candona and Cypris, males are never found, and parthenogenetic reproduction by the females appears to proceed uninterruptedly. Weismann[83] kept females of Cypris reptans breeding parthenogenetically for eight years. He also remarks on the fact that these, and indeed all parthenogenetic female Ostracoda, retain the receptaculum seminis, used normally for storing the spermatozoa derived from the male, unimpaired.
Some of the Cytheridae occur in deep water. Thus Cythere dictyon was frequently taken by the Challenger in depths of over 1000 fathoms, but the majority prefer shallow water.

Fig. 75.—Asterope oblonga, ♀, removed from its carapace, × 25. A, Alimentary canal; A1, A2, 1st and 2nd antennae; E, eye; G, gills; G.O, generative opening; H, heart; M, mandible; T, 6th appendage; T′, last appendage (cleaning foot). (After Claus.)
The Halocypridae and Cypridinidae comprise marine genera of a pelagic habit. The first antennae are chiefly sensory, but the second antennae are biramous, and they do not merely move up and down, as in the preceding families, but sideways like 109oars, the valves of the shells being excavated to admit of free movements. There are two pairs of maxillae; the succeeding limbs differ in the two families. In the Cypridinidae, e.g. Asterope (Fig. 75), the first leg (T) is lamelliform and is used as an accessory maxilla, while the second leg (T’) is turned upwards into the shell as a cleaning organ. In the Halocypridae the first leg is pediform, and differs in the two sexes, while the second leg is rudimentary and points backwards. In Asterope peculiar branchial organs (G) are present on the back. Both families possess a heart; the Halocypridae are blind, while the Cypridinidae possess eyes.
The Polycopidae and Cytherellidae are curious marine families of a pelagic habit, with biramous second antennae well adapted for swimming, and very broad. The first maxilla in the Polycopidae is also employed in swimming, while the second is modified into a branchial organ; the maxillae of the Cytherellidae are more normal in structure, but both carry branchial lamellae. The posterior limbs are altogether absent in Polycopidae, and in the Cytherellidae are only represented by the copulatory organs of the male.
CHAPTER V
CRUSTACEA (CONTINUED): MALACOSTRACA: LEPTOSTRACA—PHYLLOCARIDA: EUMALACOSTRACA: SYNCARIDA—ANASPIDACEA: PERACARIDA—MYSIDACEA—CUMACEA—ISOPODA—AMPHIPODA: HOPLOCARIDA—STOMATOPODA
SUB-CLASS II.—MALACOSTRACA.
The Malacostraca are generally large Crustacea, and they are characterised by the presence of a definite and constant number of segments composing the body. In addition to the paired eyes we can distinguish two pairs of antennae, a mandibular segment, and two maxillary segments composing the head region proper; there then follow eight thoracic segments, the limbs belonging to the anterior thoracic segments being often turned forwards towards the mouth, and modified in structure to act as maxillipedes, while at any rate the last four are used in locomotion and are termed “pereiopods.”[84] The abdomen is composed of six segments, which typically carry as many pairs of biramous “pleopods,” and the body terminates in a telson. Not counting the paired eyes or the telson, there are present nineteen segments. The excretory organs in the adult open at the bases of the second antennae, and are known as “green glands,” but in the larva maxillary glands may be present homologous to those which persist in the adult Entomostraca. This is the typical arrangement, but sometimes the maxillary glands persist in adult Malacostraca, e.g. Nebalia, Anaspides, and some Isopods.
The hepato-pancreatic diverticula are directed posteriorly, and not anteriorly as in most Entomostraca, and the stomach is often furnished with chitinous teeth and ridges forming an elaborate gastric mill, especially in the larger Decapods.
SERIES 1. LEPTOSTRACA.
Division. Phyllocarida.

Fig. 76.—Nebalia geoffroyi, ♀, × 20. A.1, A.2, 1st and 2nd antennae; Ab.1, Ab.6, 1st and 6th abdominal appendages; A.G. antennary gland; C, half of caudal fork; E, eye; G, ventral ganglionic chain; H, heart; I, intestine; L, upper liver-diverticulum; M, adductor muscle of halves of carapace; MX, palp of 1st maxilla; O, ovary; R, rostrum. (After Claus.)
The small shrimp-like Crustacean Nebalia, which is found burrowing in the superficial layers of sand in the littoral and sometimes the deeper regions of most seas, has been regarded, ever since its anatomy was made out by Claus,[85] as a connecting link between Entomostraca and Malacostraca, and has been placed in a separate group Leptostraca.
The segmentation of the body is Malacostracan, save that two extra segments are present in the abdomen, and the paired compound eyes are borne upon stalks. The eight thoracic limbs are all very similar; they are built on the typical biramous plan, and each carries a bract; they have been compared, owing to their flattened, expanded shape, to the 112foliaceous limbs of the Phyllopods. The abdominal appendages are also biramous. The heart is greatly elongated, stretching through thorax and abdomen; there are present both the antennary excretory glands characteristic of adult Malacostraca and the maxillary glands characteristic of adult Entomostraca, and both the posterior and anterior livers characteristic of the two Orders respectively are present. This combination of characters justifies the belief that Nebalia represents a primitive form, standing to some extent in an intermediate position between Entomostraca and Malacostraca, but it may be doubted if the special relationship to the Phyllopoda, claimed on the strength of the foliaceous appearance of the thoracic limbs, can be legitimately pressed.
Nebalia shows the clearest signs of relationship to the other primitive Malacostraca, and especially to the Mysidae, which it resembles not only in general form and in the essentially biramous character of its appendages, but also in many embryological points and in the similarity in development of the brood-pouch.[86]
A large number of very ancient palaeozoic fossils are known which are placed provisionally with Nebalia in the Division Phyllocarida, and some of these are no doubt closely related to the existing isolated genus. Hymenocaris from the Cambrian.
SERIES 2. EUMALACOSTRACA.
Before entering on a description of the members of this Series it is necessary to introduce and justify a new scheme of classification which has been proposed by Dr. W. T. Calman. This scheme necessitates the abandonment of the old Order Schizopoda, and also ignores the distinction which used to be considered fundamental between the sessile-eyed Crustacea (Edriophthalmata) and the stalk-eyed forms (Podophthalmata).
The old group of Schizopoda, to which Nebalia and the isolated form Anaspides, to be considered later, are undoubtedly related, represent very clearly the stem-forms from which the various branches of the Malacostracan stock diverge. No doubt they are themselves specialised in many directions, since they are a dominant group in present day seas, but their organisation is 113fundamentally of a primitive type. We see this especially in the comparative absence of fusion or reduction of the segments of the body externally and of the nervous system internally, and in the simple undifferentiated character of the trunk-limbs, all of which conform to the primitive biramous type. The most anterior thoracic limbs of the Schizopods are of particular interest. In the higher Malacostraca three of these limbs are usually turned forwards towards the mouth to act as maxillipedes, and the most anterior of all, the first maxillipede, is apt, especially in the Decapoda, to take on a flattened foliaceous form owing to the expansion of the basal segments to act as gnathobases (see Fig. 1, A, p. 10). Now this appendage in the Schizopods preserves its typical biramous character, and resembles the succeeding thoracic limbs, but in many of the species the basal joints show a tendency to be produced into biting blades (Fig. 1, E, p. 10), thus indicating the first step in the evolution of the foliaceous first maxillipede of the Decapoda. The primitive character of the Schizopods is also indicated by the fact that most of the Decapoda with uniramous limbs on the five hinder thoracic segments pass through what is known as the “Mysis stage” in development, when these limbs are biramous, the exopodites being subsequently lost in most cases.
The “Schizopoda” include a very large number of pelagic Crustacea of moderate size, which superficially appear to resemble one another very closely. The slender, elongated body, the presence of biramous limbs on all the thoracic and abdominal segments, and the possession of a single row of gills at the bases of the thoracic limbs, are, generally speaking, typical of the families Mysidae, Lophogastridae, Eucopiidae, and Euphausiidae, which go to make up the old Order Schizopoda.
It has, however, been pointed out first by Boas,[87] and subsequently by Hansen and Calman,[88] that the Euphausiidae are in many respects distinct from the other three families, and agree with the Decapoda, while the Eucopiidae, Lophogastridae, and Mysidae agree with the Cumacea, Isopoda, and Amphipoda.
It has, therefore, been suggested by these authors that the classification of the Malacostraca should be revised, and Calman (loc. cit.) has brought forward the following scheme:—
114The division Peracarida, including the Eucopiidae, Lophogastridae, and Mysidae (= Mysidacea), the Cumacea, Isopoda, and Amphipoda, is characterised by the fact that when a carapace is present it leaves at least four of the thoracic segments free and uncoalesced: by the presence of a brood-pouch formed from the oostegites on the thoracic limbs of the female: by the elongated heart: by the few and simple hepatic caeca: by the filiform spermatozoa: and by the direct method of development without a complicated larval metamorphosis. The biting face of the mandible has a movable joint, the “lacinia mobilis.”[89]
The division Eucarida, on the other hand, including the Euphausiidae and the Decapoda, shows the converse of these characters. The carapace coalesces with all the thoracic segments, there is never a brood-pouch formed from oostegites, the hepatic caeca are much ramified, the heart is short, the spermatozoa are spherical with radiating pseudopodia, the development is indirect with a complicated metamorphosis, and the mandible is without a lacinia mobilis.
Corresponding divisions are made by Calman to receive the other Malacostraca, namely, the Phyllocarida for Nebalia, the Syncarida for Anaspides, and the Hoplocarida for the Stomatopoda or Squillidae.
The important array of characters which separates the Euphausiidae from the other Schizopods and unites them with the Decapoda can no longer be neglected, and the consideration of Anaspides and its allies will further emphasise the extreme difficulty of retaining the Schizopoda as a natural group. In the sequel Calman’s proposed scheme will be adopted.
DIVISION 1. SYNCARIDA.
There is no carapace, and all the eight thoracic segments may be free and distinct. Eyes may be pedunculate or sessile. The mandible is without a lacinia mobilis. There is no brood-pouch, the eggs being deposited and hidden after fertilisation. The spermatozoa are filiform, the hepatic caeca very numerous, and the heart tubular and elongated, with ostia only in one place in 115the anterior thoracic region. The auditory organ is at the base of the first antennae.
Order. Anaspidacea.
Fam. 1. Anaspididae.—The mountain-shrimp of Tasmania, Anaspides tasmaniae, was first described by Thomson[90] in 1893 from specimens taken in a little pool near the summit of Mount Wellington; it was redescribed by Calman,[91] who drew attention to its remarkable resemblance to certain Carboniferous fossils of Europe and N. America (Gampsonyx, Palaeocaris, etc.).
The creature appears to be confined to the deep pools of the rivers and tarns on the mountains of the southern and western portions of Tasmania.[92] The waters in which it occurs are always cold and absolutely clear, and there is no record of its living at altitudes much below 2000 feet, while it frequently occurs at 4000 feet. The body may attain upwards of two inches in length; it is deeply pigmented with black chromatophores, and it is held perfectly horizontal without any flexure. The animal rarely swims unless disturbed, usually walking about on stones and water-plants at the bottom of deep pools. In walking the endopodites of the thoracic limbs are chiefly instrumental, but they are assisted by the exopodites of the abdominal limbs.
When frightened the shrimp can dart rapidly forwards or sideways by the strokes of its powerful tail-fan, but it never jumps backwards as do the other Malacostraca. It appears to browse upon the algal slime covering the rocks and on the submerged liver-worts and mosses, but it does not refuse animal food, even feeding on the dead bodies of members of its own species. The thoracic limbs, which are all biramous except the last pair, carry a double series of remarkable plate-like gills on their coxopodites. The slender and setose exopodites of the thoracic limbs are respiratory in function, being kept in continual motion even when the animal is at rest, and serving to keep up a current of fresh water round the gills.
Anaspides shows a remarkable combination of structural characters, some of which are peculiar, while others are possessed in common with the Peracarida or Eucarida. The chief peculiar 116characters are the entire absence of a carapace, and the freedom of the eight thoracic segments, with eight free thoracic ganglia in the nerve-cord; the peculiar double series of plate-like gills; the structure of the alimentary canal; and the fact that the eggs, instead of being carried in a brood-pouch, or affixed to the abdominal limbs, are deposited under stones and among water-plants.[93]

Fig. 77.—Anaspides tasmaniae in natural position for walking, × 1. The last two pereiopods point backwards and are overlapped by the first two pleopods.
The Peracaridan features, uniting it especially with the Mysidacea, are the structure of the elongated heart, the filiform spermatozoa, and the fact that no complicated metamorphosis is passed through, the young hatching out in a condition similar to, though possibly not identical with, the adult form.
The Eucaridan, especially Decapodan, features are the presence of an auditory sac on the basal joint of the antennules, 117and the modification of the endopodites of the first two abdominal appendages in the male to form a copulatory organ.
A type of a new genus of this family was found by me in the littoral zone of the Great Lake of Tasmania at an elevation of 3700 feet, and named Paranaspides lacustris.
This little shrimp (Fig. 78), which does not appear to grow to more than an inch in length, is totally different in appearance from Anaspides, being pale green and transparent, with a very marked dorsal hump as in Mysis, to which it bears a very striking superficial resemblance. It leads a more active swimming life than Anaspides, and with this habit is correlated the flexure of the body and the greater size of the tail-fan and the scale of the second antenna. The mandible is peculiar in being furnished with a four-jointed biramous palp, while that of Anaspides is three-jointed and uniramous, and the first thoracic appendage is provided with a setose biting lobe on the antepenultimate joint, thus more resembling a maxillipede. In other respects it agrees essentially in structure with Anaspides.

Fig. 78.—Paranaspides lacustris, × 4. a1, a2, First and second antennae; Ab.1, first abdominal segment; ep, epipodites or gills on the thoracic legs; md, mandible; Pl.1, first pleopod; T, telson; Th.8, eighth free thoracic segment; U, uropod, or sixth pleopod.
Fam. 2. Koonungidae.—The sole representative of this family, Koonunga cursor, has been recently described by Mr. O. A. Sayce,[94] of Melbourne University, from a small stream some 118miles to the west of Melbourne. Although plainly belonging to the Anaspidacea, this interesting little animal, which only measures a few millimetres in length, and follows a similar habit to Anaspides, running about with its body unflexed, differs from all the other members of the Division in possessing sessile instead of stalked eyes, in the first thoracic segment being fixed to the head, and in a number of minor anatomical points.
It is impossible at present to assign the Carboniferous forms (Gampsonyx, Palaeocaris, etc.) to their exact position in the Division, but it seems that they agreed more closely with Anaspides than with the other two genera. From the position in which the fossils are preserved, it would appear that they followed a similar walking habit to Anaspides, and that the body was unflexed.
DIVISION 2. PERACARIDA.
The carapace, when present, leaves at least four of the thoracic somites distinct; the first thoracic segment is always fused with the head. The eyes are pedunculate or sessile.
The mandible possesses a lacinia mobilis. A brood-pouch is formed in the female from oostegites attached to the thoracic limbs. The hepatic caeca are few and simple; the heart is elongated and tubular; the spermatozoa are filiform, and development takes place without a complicated metamorphosis.
Order I. Mysidacea.
The Mysidacea, although pelagic, are not very often met with in the true plankton on the surface; they generally swim some way below the surface, going down in many cases into the abysses. For this reason they thrive excellently in aquaria, and the common Mysis vulgaris is often present in such numbers in the tanks at the Zoological station at Naples as to damage the other inmates by the mere press of numbers. The Mysidacea, like the majority of the Peracarida, undergo a direct development, and hatch out with the structure of the adult fully formed.
Many of the Mysidacea bear auditory sacs upon the sixth pair of pleopods, a characteristic not found in the Euphausiacea.
Fam. 1. Eucopiidae.—The curious form Eucopia australis 119(Fig. 79) described by Sars,[95] may be chosen as an example of the Mysidacea.
The peculiarity of this form consists chiefly in the immense elongation of the endopodites of the fifth, sixth, and seventh thoracic appendages. Characteristic of the Mysidacea is the freedom of the hinder thoracic segments from fusion with the carapace, otherwise this animal is seen closely to resemble the Euphausia figured (Fig. 102). Eucopia australis, like so many of the Mysidacea, is a deep-sea animal, being brought up with the dredge from over 1000 fathoms; it is very widely distributed over the Atlantic Ocean.

Fig. 79.—Eucopia australis, young female, × 3. A, 1st antenna; Ab.1, 1st abdominal segment; Ab.6, 6th abdominal appendage; E, eye; T, telson; Th, 5th thoracic appendage. (After Sars.)
Fam. 2. Lophogastridae.—The members of this family (Lophogaster, Gnathophausia) agree with the Eucopiidae in the possession of branched gills on some of the thoracic limbs, in the absence of auditory sacs on the sixth pair of pleopods, in the presence of normally developed pleopods in both the male and female, and in the brood-lamellae being developed on all seven of the thoracic limbs. The endopodites of the posterior thoracic limbs are, however, of a normal size.

Fig. 80.—Dorsal view of male Diastylis stygia, × 12. A, 2nd antenna; Ab.6, 6th abdominal appendage. (After Sars.)
Fam. 3. Mysidae.—These differ from both the foregoing families in the absence of gills, in the presence of an auditory sac on the sixth pleopods, in the reduction of the other pleopods in the female, and in the brood-lamellae being developed only on the more posterior pairs of thoracic limbs. A number of closely 120related genera compose this family, of which Mysis, Boreomysis, and Siriella may be mentioned. Mysis oculata, var. relicta, is a fresh-water form from the lakes of northern and central Europe.
Order II. Cumacea.[96]
The Cumacea are a group of small marine animals rarely attaining an inch in length, which agree with the Mysidacea in the characters noted above as diagnostic of the Division Peracarida; they possess, however, in addition a number of peculiar properties, and Sars believes them to be of a primitive nature showing relationship to Nebalia, and possibly to an ancestral Zoaea-like form. They follow a habit similar to that of the Mysidacea, being caught either in the surface-plankton or in great depths, many of the deep-sea forms being blind. They are, however, not true plankton forms, and they appear to attain a greater development both in point of variety and size in the seas of the northern hemisphere. The thoracic limbs may be biramous, but there is a tendency among many of the genera to lose the exopodites of some of the thoracic legs, an exopodite never being present on the last few thoracic limbs of the female and on the last in the male. In the Cumidae the four posterior pairs in both sexes have no exopodites. The first three thoracic appendages following the maxillae are distinguished as maxillipedes; they are uniramous, and the first pair carries an 121epipodite and a large gill upon the basal joints. Pleopods are only developed in the male sex.
The flagellum of the second antennae in the male may be enormously elongated, as in the Atlantic deep-sea species shown in Fig. 80, so as to exceed in length the rest of the body.
Fam. 1. Cumidae.—No sharp demarcation between thorax and abdomen. Four posterior pairs of legs in both sexes without exopodites. Male with five well-developed pleopods in addition to the uropods. Telson wanting. Cuma, Cyclaspis, etc.
Fam. 2. Lampropidae.—Body-form resembles that of Cumidae. All the thoracic limbs except the last have exopodites. The male has three pairs of pleopods. Telson present. Lamprops, Platyaspis, etc.
Fam. 3. Leuconidae.—Body-form similar to above. Male has only two pairs of pleopods. Mouth-parts peculiar, much less setose than in other families. Telson absent. Leucon, Eudorella.
Fam. 4. Diastylidae.—Anterior part of thorax sharply marked off from posterior part. Male has two pairs of pleopods. Telson present. Diastylis (Fig. 80). D. goodsiri from the Arctic ocean measures over an inch in length.
Fam. 5. Pseudocumidae.—Rather similar to Diastylidae, but differ in reduced size of telson and presence of exopodites on third and fourth thoracic legs of female. This family is represented by three very similar marine forms of the genus Pseudocuma; but, as Sars has shown,[97] the Caspian Sea contains thirteen peculiar species, only one of which can be referred to the genus Pseudocuma, while the rest may be partitioned among four genera, Pterocuma, Stenocuma, Caspiocuma, Schizorhynchus.
Order III. Isopoda.
The Isopoda and the Amphipoda are frequently classed together as Arthrostraca or Edriophthalmata, owing to a number of features which they share in common, as, for instance, the sessile eyes which distinguish them from the podophthalmatous Schizopoda and Decapoda, the absence of a carapace, and the thoracic limbs which are uniramous throughout their whole existence. For the rest, in the presence of brood-plates and the other diagnostic 122characters, they are plainly allied to the other Peracarida, and an easy transition is effected from the Mysidacea to the Isopoda through the Chelifera or Anisopoda. Only one thoracic segment is usually fused with the head, the appendage of this segment being the maxillipede; in the Chelifera among Isopoda, and the Caprellidae among Amphipoda, two thoracic segments are fused with the head.
The Isopoda are distinguished from the Amphipoda by the dorso-ventral flattening of the body, as opposed to the lateral flattening in the Amphipoda, by the posterior position of the heart, and by the branchial organs being situated on the abdominal instead of on the thoracic limbs.
The Isopoda, following Sars’[98] classification, fall into six sub-orders—the Chelifera, Flabellifera, Valvifera, Asellota, Oniscoida, and Epicarida,—to which must be added the Phreatoicidea.
Sub-Order 1. Chelifera.
The Chelifera, including the families (1) Apseudidae and (2) Tanaidae, are interesting in that they afford a transition between the ordinary Isopods and the Mysidacea. The important features in which they resemble the Mysidacea are, first, the fusion of the first two thoracic segments with the head, with the coincident formation of a kind of carapace in which the respiratory functions are discharged by a pair of branchial lamellae attached to the maxillipedes; and, second, the presence of very small exopodites on the first two thoracic appendages of the Apseudidae.
The second pair of thoracic limbs, i.e. the pair behind the maxillipedes, are developed both in the Apseudidae and Tanaidae into a pair of powerful chelae, and these frequently show marked sexual differences, being much more highly developed in the males than in the females. The biramous and flattened pleopods are purely natatory in function, and the uropods or pleopods of the sixth pair are terminal in position and slender.

Fig. 81.—Apseudes spinosus, ♂, × 15. A, 1st antenna; Ab, 6th abdominal appendage; T, 2nd thoracic appendage. (After Sars.)
Both families, of which the Apseudidae contain the larger forms, sometimes attaining to an inch in length, are littoral in habit, or occur in sand and ooze at considerable depths, many of the genera being blind. Many Tanaids (e.g. Leptochelia, Tanais, Heterotanais, etc.) live in the algal growths of the littoral zone, and being highly heliotropic they are easy to collect if a basinful of algae is placed in a strong light. The females carry the eggs about with them in a brood-pouch formed, as is usual in the Peracarida, by lamellae produced from the bases of the thoracic limbs. The males on coming to maturity do not appear to grow any more, or to take food, their mouth-parts frequently degenerating and the alimentary canal being devoid of food. They are thus in the position of insects which do not moult after coming to maturity; and, as in Insects, the males are apt to show a kind of high and low dimorphism—certain of the males being small with secondary sexual characters little different from those of the females, while others are large with these characters highly developed. Fritz Müller, in his Facts for Darwin, observes that in a Brazilian species of Leptochelia, apparently identical with the European L. dubia, the males occur under two totally distinct forms—one in which the chelae are greatly developed, and another in which the chelae resemble those of the female, but the antennae in this form are provided with far longer and more numerous sensory hairs than in the first form. Müller suggested that these two varieties were produced by natural selection, the characters of the one form compensating for the absence of the characters of the other. A general consideration of the sexual dimorphism in the Tanaidae[99] 124lends some support to this view, since the smaller species with feeble chelae do appear to be compensated by a greater development of sensory hairs on the antennae, but the specific differences are so difficult to appreciate in the Tanaidae that it is possible that the two forms of the male in Müller’s supposed single species really belonged to two separate species.
Sub-Order 2. Flabellifera.
The Flabellifera include a number of rather heterogeneous families which resemble one another, however, in the uropods being lateral and not terminal, and being expanded together with the telson to form a caudal fan for swimming. The pleopods are sometimes natatory and sometimes branchial in function. Some of the families are parasitic or semi-parasitic in habit.
Fam. 1. Anthuridae.—These are elongated cylindrical creatures found in mud and among weeds upon the sea-bottom; their mouth-parts are evidently intended for piercing and sucking, but whether they are parasitic at certain periods on other animals is not exactly known. Anthura, Paranthura, Cruregens.

Fig. 82.—Gnathia maxillaris. A, Segmented larva, × 10; B, Praniza larva, × 5; C, gravid female, × 5; D, male, × 5.
Fam. 2. Gnathiidae.[100]—These forms appear to be related to the Anthuridae; they are ectoparasitic on various kinds of fish during larval life, but on assuming the adult state they do not feed any more, subsisting merely on the nourishment amassed during the larval periods. The larvae themselves are continually leaving their hosts, and can be taken in great numbers living freely among weeds on the sea-bottom. The larvae, together with the adults of Gnathia maxillaris, are extremely abundant among the roots of the sea-weed Poseidonia cavolinii in the Bay of Naples. The young larvae hatch out from the body of the female in the state shown in Fig. 82, A. This minute larva fixes upon a fish, and after a time it is transformed into the so-called Praniza larva (B), in which the gut is so distended with the fluid sucked from the host that the segmentation in the hind part of the thorax is entirely lost. When this larva moults it may, however, reacquire temporarily its segmentation. After a certain period of this parasitic mode of life the Praniza finally abandons its host, and becomes transformed into the adult male or female. This may take place at very different stages in the 125growth of the larva, the range of variation in size of the adults being 1–8 mm., and it must be remembered that when once the adult condition is assumed growth entirely ceases. What it is that determines the stage of growth in each individual when it shall be transformed into the adult is not known. The males and females differ from one another so extraordinarily that it was for long denied that they were both derived from the Praniza larvae. This is nevertheless the case. The change from the Praniza to the female (Fig. 82, C) is not very great. The ovary absorbs all the nourishment in the gut and comes to occupy the whole of the body, all the other organs degenerating, including the alimentary canal and mouth-parts. Indeed, only the limbs with their muscles and the nervous system remain. The change to the male (D) is more radical. The food is here stored in the liver, which increases in the male just as the ovary does in the female. The segmentation is reacquired, and the massive square head is formed from the hinder part of the head in the Praniza, the anterior portion with its stylet-like appendages being thrown away. The powerful nippers of the male are not formed inside the cases of the old styliform mandibles, but are independent and possibly not homologous organs. The meaning of the marked sexual dimorphism and the use of the males’ nippers are not in the least known, though the animals are easy to keep under observation. In captivity the males never take the slightest notice of either larval or adult females.
126Fam. 3. Cymothoidae.[101]—This is a group of parasites more completely parasitic than the foregoing, but their outer organisation does not differ greatly from an ordinary Isopodan form. A great many very similar species are known which infest the gill-chambers, mouths, and skin of various fishes. The chief interest that attaches to them is found in the fact that a number of them, and perhaps all, are hermaphrodite, each individual acting as a male when free-swimming and young, and then subsequently settling down and becoming female. This condition is exactly the same as that occurring universally in the great group of parasitic Isopoda, the Epicarida, to be considered later. There is no evidence that the Cymothoidae are phyletically related to the Epicarida, so that the similar sexual organisation appears to be due to convergence resulting from similar conditions of life. The general question of hermaphroditism in the Crustacea has been shortly discussed on pp. 105–106. Cymothoa.
Fam. 4. Cirolanidae.—In this family is placed the largest Isopod known—the deep-sea Bathynomus giganteus, found in the Gulf of Mexico and the Indian Ocean, sometimes measuring a foot long by four inches broad. A common small littoral form is Cirolana.
Fam. 5. Serolidae.[102]—The genus Serolis comprises flattened forms bearing a curious resemblance to Trilobites, which Milne Edwards considered more than superficial. The genus is confined to the littoral and deep waters of the southern hemisphere.
Fam. 6. Sphaeromidae.[103]—These are flattened, broad-bodied forms, most commonly met with in the Mediterranean and warmer seas. Without being actually parasitic, they are frequently found as scavengers in decaying material, and they show some relationship to the parasitic Cymothoidae. In some of the genera, e.g. Cymodoce, the ovigerous female shows a degenerate condition 127of the mouth-parts, while the maxillipedes undergo an enlargement, and are used for causing a current through the brood-chamber.
Sub-Order 3. Valvifera.

Fig. 83.—Munnopsis typica (Munnopsidae), ♂, × 2. A, 2nd antenna; Ab, abdomen; T, 5th thoracic appendage or 4th leg. (After Sars.)
The Valvifera, illustrated by the Idotheidae and Arcturidae, are characterised by the uropods being turned back and expanded to form folding doors covering up the delicate pleopods, which are mostly respiratory in function, though the anterior pairs may serve as swimming organs. Arcturus is a typically deep sea genus, many species, remarkably furnished with spiny processes, having been taken by the Challenger in the southern hemisphere. The Idotheidae are more littoral forms, several species of Idothea being commonly met with off the British coasts, occasionally penetrating into brackish or even fresh water.
Sub-Order 4. Asellota.
In this group the abdominal segments are fused dorsally to form a shield-like caudal region; the pleopods are respiratory in function and reduced in numbers, the first pair being often expanded and produced backwards to form an operculum covering the rest. Several of the Asellota are fresh-water, Asellus aquaticus 128(Asellidae) being extremely abundant all over Europe in weed-grown ditches, the mud of slowly-moving streams, and even on the shores of large lakes. They are mostly sluggish in habit, but the marine Munnopsidae (Fig. 83, Munnopsis) are expert swimmers, the swimming organs being fashioned by the expansion and elongation of the thoracic legs.
Sub-Order 5. Oniscoida.
The Oniscoida[104] are terrestrial forms in which the abdomen is fully segmented, the pleopods are respiratory, their endopodites being delicate branchiae, while their exopodites are plate-like and form protective opercula for the gills, and the uropods are biramous and not expanded. The epimera of the segments are greatly produced. The terrestrial Isopods, although air-breathers,[105] are dependent on moisture, and are only found in damp situations. It seems probable that they have been derived from marine Isopods, since the more generalised of them, e.g., Ligia (Fig. 84), common on the English coasts, are only found in damp caves and crannies in the rocks.

Fig. 84.—Ligia oceanica, ventral and dorsal views, × 1. (From original drawings prepared for Professor Weldon.)
129The related Ligidium is found far inland, but always in the neighbourhood of water. These two genera may be distinguished by the numerous joints in the flagellum of the second antennae, the flagellum being in all cases the portion of the antenna succeeding the long fifth joint. Philoscia muscorum occurs usually near the coast, but it is also found inland in England under trees in damp moss. This genus and the common Oniscus, found in woods, are distinguished by the presence of three joints in the flagellum of the second antenna. Philoscia can be distinguished from Oniscus by its narrower body and the pretty marbled appearance of its back. The genus Trichoniscus has four joints in the flagellum; various species are found in woods. In Porcellio and Armadillidium there are only two joints in the flagellum, while Armadillidium, the common garden wood-louse, can be distinguished from all others by the flattened shape of the uropods, and the habit of rolling up into a ball like an Armadillo.
There is also a very peculiar species, Platyarthrus hoffmannseggii, which occurs in England and Northern Europe, and always lives in ants’ nests. It is supposed that they serve as scavengers for the ants, which tend them carefully, and evidently treat them as domestic animals of some kind. The small creature is quite white and blind, and has exceedingly short antennae.
Sub-Order 6. Epicarida.
The Epicarida include an immense number of Isopods, parasitic upon other Crustacea. In the adult state they become greatly deformed, and offer very few characters of classificatory value, but they all pass through certain highly characteristic larval stages which are essentially similar in the different families. All the species are protandric hermaphrodites, each individual being male while in a larval state, and then losing its male organisation and becoming female as the parasitic habit is assumed.
Two series of families are recognised according to the larval stages passed through, the Cryptoniscina, in which the adult male organisation is assumed in the Cryptoniscus stage, and the female condition is imposed directly upon this form, and the Bopyrina, in which the Cryptoniscus passes into a further larval stage, the Bopyrus, which performs the function of the 130male, and upon which the female organisation is imposed as the parasitic habit is assumed.
The following is a list of the Epicarida with the Crustacea which serve as their hosts[106]:—
| Cryptoniscina | Microniscidae | on Copepoda. |
| Cryptoniscidae | on Ostracoda. | |
| Liriopsidae | on Rhizocephala. | |
| Hemioniscidae | on Cirripedia. | |
| Cabiropsidae | on Isopoda. | |
| Podasconidae | on Amphipoda. | |
| Asconiscidae | on Schizopoda. | |
| Bopyrina | Dajidae | on Decapoda |
| Phryxidae | ||
| Bopyridae | ||
| Entoniscidae | ||

Fig. 85.—Epicaridian larva, probably belonging to one of the Cryptoniscina. A, 2nd antenna; Ab, abdominal appendages; T, thoracic appendages. (From Bonnier, after Hansen.)
In all cases the first larval form which hatches out from the maternal brood-pouch is called the Epicaridian larva (Fig. 85).
This little larva has two pairs of antennae, a pair of curious frontal processes, and a pair of mandibles. The other mouth-parts are missing; there are only six thoracic limbs, but the full complement of six biramous pleopods are present, and at the end of the body there may be a long tube of unknown function.
As a type of the Cryptoniscina we may take the Liriopsidae,[107] parasitic on the Rhizocephala, which are, of course, themselves parasitic on the Decapoda, the whole association forming a very remarkable study in Carcinology.
Almost every species of the Rhizocephala is subject to the attacks of Liriopsids, the latter fixing either on the Rhizocephala themselves, or else on the Decapod host at a point near the fixation of the Rhizocephalous parasite. An exceedingly common Liriopsid is Danalia curvata, parasitic on Sacculina neglecta, 131which is itself parasitic on the spider-crab, Inachus mauritanicus, at Naples. The adult Danalia is a mere curved bag full of eggs or developing embryos, and without any other recognisable organs except two pairs of spermathecae upon the ventral surface where the spermatozoa derived from the larval males are stored.

Fig. 86.—Inachus mauritanicus, ♀, × 1, carrying two Sacculina neglecta (a, b), and a Danalia curvata (c), the latter bearing two dwarf males.
In Fig. 86 is represented a female of Inachus mauritanicus which carried upon it two Sacculinae and a Danalia curvata, and upon the latter are seen two minute larval males in the act of fertilising the adult Danalia. The eggs develop into the Epicaridian stage, after which the larva passes into the Cryptoniscus stage (Fig. 87). In this larval form the segments are clearly delimited; the only mouth-parts present are the mandibles, but there are seven pairs of thoracic limbs and the full number of pleopods. This Cryptoniscus stage is found in all the Epicarida, and only differs in detail in the various families.

Fig. 87.—Ventral view of Cryptoniscus larva of Danalia curvata, ♂, × 25.
In the Cryptoniscina the Cryptoniscus larva is the male, and at this stage possesses a pair of large testes in the thorax. The ovaries are also present at this stage as very small bodies applied to the anterior ends of the testes. The larval males in this state seek out adult fixed Danaliae and fertilise them; and, when this is accomplished, they themselves become fixed to the host and begin to develop into the adult female condition. The limbs are all lost, and out of the mouth grows a long proboscis (Fig. 88, P), which penetrates the tissues of the host. The ovaries begin to grow, and a remarkable process of absorption in the testes takes place. These organs, when fixation occurs, are never empty of spermatozoa, and are frequently crammed with them. After fixation some 132large cells at the interior borders of the testes begin to feed upon the remains of these organs and to grow enormously in size and to multiply by amitosis. These phagocytes, as they really are, attain an enormous size, but they are doomed to degeneration, the chromatin becoming dispersed through the cytoplasm, and the nuclei dividing first by amitosis and then breaking up and disappearing. As the parasite grows, the heart at the posterior end of the body ceases to beat; the ovaries increase enormously at the expense of the alimentary canal, and on the ventral surface two pairs of spermathecae are invaginated ready to receive the spermatozoa of a larval male. In the adult condition, after fertilisation has taken place and the ovaries occupy almost the whole of the body, the remains of the phagocytic cells can be seen on the dorsal surface in a degenerate state. They evidently are not used as food, and their sole function is to make away with the male organisation when it has become useless.[108]

Fig. 88.—Side view of Danalia curvata, × 15, shortly after fixation and loss of larval appendages. A, Alimentary canal; E, eye; H, heart; N, phagocytic cells; O, ovary; P, proboscis.

In the series Bopyrina, after the free-living Epicaridian 133and Cryptoniscus stages, a further larval state is assumed, called the Bopyrus, which is the functional male, and, after performing this function, passes on to the adult female condition.
The family Bopyridae is parasitic in the branchial chamber of Decapoda, especially Macrura and Anomura. When one of these Decapods is infested with an adult Bopyrid the gill-chamber in which it is situated is greatly swollen, as shown in Fig. 90. A very common Bopyrid is Bopyrus fougerouxi, parasitic in the gill-chambers of Palaemon serratus. The Bopyrus larva or functional male has the appearance shown in Fig. 91. It differs from the Cryptoniscus stage in possessing a rudimentary pair of anterior thoracic limbs and seven pairs normally developed, while the abdominal limbs are plate-like and branchial in function. The male can often be found attached to the female beneath the last pair of incubatory lamellae.

Fig. 90.—Galathea intermedia, with a Pleurocrypta microbranchiata under its left branchiostegite (B), × 1. (After Sars.)

Fig. 91.—Ventral view of male Bopyrus fougerouxi, × 30. A, 1st and 2nd antennae; T, 8th (last) thoracic appendage. (After Bonnier.)
The adult female condition, which is assumed after the Bopyrid stage is passed through, is illustrated in Fig. 92. The body acquires a remarkable asymmetry, due to the unequal pressure exerted by the walls of the gill-chamber. The antennae and mandibles (Fig. 92, B) are entirely covered up by the largely expanded maxillipedes; maxillae are, as usual, entirely 134absent. Very large lamellae grow out from the bases of the thoracic limbs to form a brood-pouch, and in this manner the adult condition is attained.
The final complication in the life-histories of these Isopoda is reached by the family Entoniscidae, which are parasitic when adult inside the thoracic cavity of Brachyura and Paguridae. The cephalothorax of a Carcinus maenas, which contains an adult Portunion maenadis (P), is shown in Fig. 93. The parasite is of a reddish colour when alive.

Fig. 92.—Bopyrus fougerouxi. A, Ventral view of female carrying a male (M) between her abdominal appendages, × 8; B, ventral view of part of head of female, the maxillipedes and the left mandible having been removed. A.1, A.2, 1st and 2nd antennae; M, male; Mn, right mandible; Mx, left maxillipede; O, oostegite; T, left 4th thoracic appendage or 3rd leg. (After Bonnier.)

Fig. 93.—Cephalothorax of Carcinus maenas, seen from the ventral side, containing a parasitic Portunion maenadis (P), × ½. (After Bonnier.)
The Entoniscidae pass through a free living Epicaridian and Cryptoniscus stage, and become adult males in the Bopyrus stage. It is stated, however, by Giard and Bonnier[109] that these individuals, which actually function as males, never grow up 135into adult females, though all the adult females have passed through a male stage in which the male genital ducts are not formed. The hermaphroditism, therefore, in these animals at any rate is absolutely useless from a reproductive point of view, and this justifies our looking for some other explanation of it, such as was suggested on p. 105.

Fig. 94.—Portunion maenadis, ♀:—A, Young, × 10; B, older, × 5; C, adult, before the eggs are laid, × 3. A, 2nd antenna; Ab, abdomen; B, anterior lobe of brood-pouch; B′, its lateral lobe; H, head; 1, 2, 1st and 2nd incubatory lamellae (oostegites). (After Giard and Bonnier.)
The Bopyrus fixes in the gill-chamber of the host and becomes converted into the adult female by a series of transformations. As these changes take place it invaginates the wall of the gill-chamber and pushes its way into the thoracic cavity of the crab, though it lies all the time enveloped in the invaginated wall of the gill-chamber, and not free in the body-cavity of the crab. The transformations which it undergoes are shown in Fig. 94. The body first assumes a grub-like appearance (A), and two pairs of incubatory lamellae (1, 2) grow out from the first and second thoracic segments. In the next stage (B) these lamellae assume gigantic proportions, and four pairs of branchiae grow out from 136the abdominal segments (Ab). In the final stage (C) the incubatory lamellae have further increased in size, and constitute the main bulk of the body; the enormous mass of eggs is passed into the incubatory pouch, and all that remains of the rest of the body is the small head (H) and the abdomen (Ab), furnished with its branchiae. Communication with the external world is kept up through an aperture which leads from the brood-pouch into the gill-chamber of the host, and through this aperture the young are hatched out when they are developed sufficiently.
The presence of these parasites, although they are never in actual contact with the internal organs of the crab, calls forth the same phenomenon of parasitic castration as was observed in the Rhizocephala. A remarkable association is also found to exist between the Entoniscidae and Rhizocephala, of such a kind that, on the whole, a crab infested with a Rhizocephalan is more likely to harbour an Entoniscid than one without. The explanation of this association is probably that a crab with a Sacculina inside it is prevented from moulting as often as an uninfected crab, and, in consequence, the larval stages of the Entoniscid in the crab’s gill-chamber are more safely passed through.
Sub-Order 7. Phreatoicidea.[110]
The members of this sub-order, although agreeing with the Isopoda in the essentials of their anatomy, resemble the Amphipoda in being rather laterally compressed, and in having the hand of the first free thoracic limb enlarged and subchelate. The abdomen is greatly produced laterally by expansions of the segments. In fact, the shape of the body and of the limbs is very Amphipodan.—Phreatoicus from New Zealand, Southern Australia, and Tasmania. Phreatoicopsis,[111] a very large form from Gippsland, Victoria. Only one family exists, Phreatoicidae.
Order IV. Amphipoda.
In this order the body is flattened laterally, the heart is anterior in position, and the branchial organs are attached to the thoracic limbs.
There are three well defined sub-orders, (i.) the Crevettina, including 137a vast assemblage of very similar animals, of which the common Gammarus and Orchestia may serve as examples; (ii.) the Laemodipoda or Caprellids, and (iii.) the Hyperina.
We cannot do more than touch on the organisation of these sub-orders.
Sub-Order 1. Crevettina.
In this sub-order only one thoracic segment is fused with the head; the basal joints of the thoracic limbs are expanded to form broad lateral plates, and the abdomen is well developed, with six pairs of pleopods, the last three pairs being always turned backwards, and stiffened to act as uropods.
This group has numerous fresh-water representatives, e.g. Gammarus of several species, the blind well-shrimp Niphargus, and the S. American Hyalella; but the vast majority of the species are marine, and are found especially in the littoral zone wherever the rocks are covered with a rich growth of algae, Polyzoa, etc. The Talitridae or “Sand-hoppers” have deserted the waters and live entirely in the sand and under rocks on the shore, and one common European species, Orchestia gammarellus, penetrates far inland, and may be found in gardens where the soil is moist many miles from the sea.
The Rev. T. R. R. Stebbing, in his standard work[112] on this group, recognises forty-one families, and more than 1000 species, so that we can only mention a few of the families, many of which, indeed, differ from one another in small characters.
Fam. Lysianassidae.—The first joint of the first antenna is short, with an accessory flagellum. Mandible with a palp, and with an almost smooth cutting edge. The third joint of the second gnathopod is elongated. This family is entirely marine, comprising forty-eight genera, with species distributed in all seas. One genus, Pseudalibrotus, inhabits the brackish water of the Caspian Sea. Lysianassa has several common British and Mediterranean species.
Fam. Haustoriidae.—The members of this family are specially adapted for burrowing, the joints of the hinder thoracic limbs being expanded, and furnished with spines for digging. Some of the species are common on the British coasts, e.g. Haustorius arenarius. Pontoporeia has an interesting distribution, 138one species, P. femorata, being entirely marine, in the Arctic and North Atlantic, P. affinis inhabiting the Atlantic, and also fresh-water lakes in Europe and North America, P. microphthalma being confined to the Caspian Sea, and P. loyi to Lakes Superior and Michigan.
Fam. Gammaridae.—Includes fifty-two genera. The first antennae are slender, with the accessory flagellum very variable. The mandibles have a dentate cutting edge, spine-row, and molar surface, and a three-jointed palp. The first two thoracic limbs are subchelate. This family includes a few marine, but mostly brackish and fresh-water species. Crangonyx is entirely subterranean in habitat, as is Niphargus, N. forelii occurring, however, in the deep waters of Lake Geneva. Both these genera are blind. Gammarus has thirty species, G. locusta being the common species on the North Atlantic coasts, and G. pulex the common fresh-water species of streams and lakes in Europe. A number of Gammaridae inhabit the Caspian Sea, e.g. Boeckia, Gmelina, Niphargoides, etc., while the enormous Gammarid fauna of Lake Baikal, constituting numerous genera, showing a great variety of structure, some of them being blind, belong to this family, e.g. Macrohectopus (Constantia), Acanthogammarus, Heterogammarus, etc.

Fig. 95.—Gammarus locusta, ♂ (above) and ♀ (below), × 4. Abd.1, First abdominal segment; T, telson; Th, seventh free thoracic segment (= 8th thoracic segment); U, third uropod. (After Della Valle.)
139Fam. Talitridae.—This family may be distinguished by the absence of a palp on the mandible, and by one ramus of the uropods being very small or wanting. The various kinds of “Sand-hoppers” belong here, familiar creatures on every sandy coast between tide-marks. The genera Talitrus and Talorchestia always frequent sand, while Orchestia is generally found under stones and among weed. Some species of Orchestia, e.g. O. gammarellus, live inland in moist places at some distance from the sea; one species of Talitrus (T. sylvaticus) occurs at great elevations in forests in Southern Australia.
Hyale is a coastal genus, and is also found on floating objects in the Sargasso Sea. Hyalella is confined to Lake Titicaca and the fresh waters of South America. Chiltonia from S. Australasia.
Fam. Corophiidae.—The members of this family have a rather flattened body and small abdomen, and the side-plates on the thorax are small. The uropods are also small and weak. Some species of the genus Corophium are characteristic of the Caspian Sea.
Sub-Order 2. Laemodipoda.

Fig. 96.—Caprella grandimana, × 4. a, Abdomen; g, gills; t, 3rd (first free) thoracic segment; t′, 8th thoracic segment. (After P. Mayer.)
Fam. 1. Caprellidae[113] are also chiefly littoral forms, swarming among rocks covered by algae, though they are by no means so easy to detect as the Gammaridae and Tanaidae which haunt similar situations. In a basinful of algae or Polyzoa taken from the rocks fringing the Bay of Naples, the latter are easily collected, the Tanaidae always crawling out of the weeds in the direction of the light, while the Gammarids dart about in all directions; but the Caprellidae, with their branching stick-like forms, 140harmonise so well with their surroundings that it requires an experienced eye to detect them. The body is elongated and thin, resembling that of a stick-insect. The first two thoracic segments are more or less completely fused with the head; the second and third thoracic limbs end in claws; the two following thoracic limbs are normal in the genus Proto, rudimentary in Protella, and absent in the remaining genera, though their gills remain as conspicuous flabellate structures. The three hind legs are normal, and the abdomen is reduced to a tiny wart at the hind end of the greatly elongated thorax.
P. Mayer has described cases of external hermaphroditism as being fairly common in certain species, e.g. Caprella acutifrons, and this is interesting if we take into consideration the frequent partial hermaphroditism exhibited by the gonad of Orchestia at certain times of year (see p. 104).
Fam. 2. Cyamidae.—These are closely related to the Caprellidae in the form of the limbs and the reduced state of the abdomen. Cyamus ceti, which lives ectoparasitically on the skin of whales, has the body expanded laterally instead of being elongated, as in the Caprellids.
Sub-Order 3. Hyperina.

Fig. 97.—Phronima sedentaria, ♀, in a Pyrosoma colony, × 1. (After Claus, from Gerstaecker and Ortmann.)
These are an equally distinct and curious group of Amphipods, characterised by the large size of the head and the transparency of the body. Instead of haunting the littoral zone they are pelagic in habit, and many of them live inside transparent pelagic Molluscs, Tunicates, or Jellyfish. A well known form is Phronima sedentaria, which inhabits the glassy barrel-like cases of the Tunicate Pyrosoma in the Mediterranean. The female is often taken in the plankton together with her brood in one of these curious glass houses; the zooids of the Pyrosoma colony are completely eaten away and the external surface of the case, instead of being rough with the tentacles of the zooids, is worn to a smooth, glass-like surface. It has been observed that the female actively navigates her house upon the surface of the sea; she clings on with her 141thoracic legs inside, while the abdomen is pushed out through an opening of the Pyrosoma case behind, and by its alternate flexion and extension drives the boat forwards, the water being thus made to enter at the front aperture and supply the female and her brood with nourishment.
DIVISION 3. HOPLOCARIDA.
The carapace leaves at least four of the thoracic somites distinct. The eyes are pedunculate. The mandibles are without a lacinia mobilis; there are no oostegites, the eggs being carried in a chamber formed by the maxillipedes. The hepatic caeca are much ramified, the heart is greatly elongated, stretching through thorax and abdomen, with a pair of ostia in each segment. The spermatozoa are spherical, and there is a complicated and peculiar metamorphosis.
Order. Stomatopoda.

Fig. 98.—Lateral view of Squilla sp., × 1. A.1, A.2, 1st and 2nd antennae; Ab.1, 1st abdominal segment; Ab.6, 6th abdominal appendage; C, cephalothorax, consisting of the head fused with the first five thoracic segments; E, eye; M, 2nd maxillipede; T, telson. (After Gerstaecker and Ortmann.)
The Stomatopoda are rather large animals, occasionally reaching a foot in length, all of which exhibit a very similar structure; Squilla mantis and S. desmaresti are found on the south coast of England not very frequently; but they are very common in the Mediterranean, living in holes or in the sand within the littoral zone of shallow water. They differ from all the other Malacostraca 142by a combination of characters, and Calman proposes the term Hoplocarida for a division equivalent to the Peracarida, Eucarida, etc.
The abdomen is very broad and well developed, ending in a widely expanded telson. There is a carapace which covers the four anterior thoracic segments, leaving the four posterior segments free. The portion of the head carrying the stalked eyes constitutes an apparently separate segment articulated to the head. The antennae, mandibles, and maxillae are normal; there then follow five pairs of uniramous thoracic limbs turned forwards as maxillipedes and ending in claws; the second pair of these is modified into a huge raptorial arm, exactly resembling that of a Praying Mantis (cf. vol. v. p. 242), by means of which the Squilla seizes its prey. The last three thoracic limbs are small and biramous. The pleopods are powerful, flattened, biramous swimming organs with small hooks or “retinaculae” upon their endopodites, which link together each member of a pair in the middle, and with large branching gills upon the exopodites.
The internal anatomy exhibits several primitive features. The nervous system is not at all concentrated, there being a separate ganglion for each segment; and the heart stretches right through thorax and abdomen, with a pair of ostia in each segment. There are also ten hepatic diverticula given off segmentally from the intestine.
The female has the curious habit of carrying the developing eggs in a chamber improvised by the apposition of the maxillipedes, so that it looks rather as if she were in the act of devouring her own brood.
The metamorphosis of the larvae, despite the work of Claus[114] and Brooks,[115] is not very accurately known, especially uncertain being the identification of the different larvae with their adult forms. The chief interest consists in the fact that certain of the anterior thoracic limbs develop in their normal order and degenerate, to be reformed later, just as in the Phyllosoma larva of the Loricata (see pp. 165, 166).
In one series of larvae, probably not of Squilla itself, but of related genera, the young hatch out as “Erichthoidina” (Fig. 99), 143with the thoracic appendages developed as biramous organs as far as the fifth pair, and with a single abdominal pair of limbs.

Fig. 99.—Erichthoidina larva of a Stomatopod, with five pairs of maxillipedes, and the first pair of abdominal appendages, × 10. (From Balfour, after Claus.)
The abdominal series of limbs is next completed; the second thoracic limb assumes its adult raptorial structure, but the succeeding three limbs become greatly reduced and may entirely degenerate, leaving the posterior six thoracic segments without limbs.
Usually the anterior three pairs are only reduced, and then redevelop side by side with the small posterior limbs as they appear. This larva is then termed the “Erichthus” (Fig. 100); but when they completely disappear the larva is called a “Pseudozoaea,” owing to its resemblance to the Zoaea stage of the Decapoda, which is also characterised by the suppressed development of the thoracic segments.

Fig. 100.—Older Erichthus larva, with six pairs of abdominal appendages, × 15. (From Balfour, after Claus.)
The so-called “Alima” larva of Squilla is also a Pseudozoaea, but it is apparently arrived at directly without the previous formation and degeneration of the anterior thoracic limbs, the larva hatching out from the egg in the Pseudozoaeal stage.
Fam. Squillidae.—Of the six known genera none extend into the cold subarctic seas; the majority are characteristic of the warm or tropical seas (Gonodactylus), some of the species having very wide ranges, e.g. G. chiragra, which is completely circumtropical, and appears to have entered the Mediterranean at some period, though it is very rare there.
CHAPTER VI
CRUSTACEA (CONTINUED)—EUMALACOSTRACA (CONTINUED): EUCARIDA—EUPHAUSIACEA—COMPOUND EYES—DECAPODA
DIVISION 4. EUCARIDA.
The carapace fuses with all the thoracic segments. The eyes are pedunculate. The mandible is without a lacinia mobilis. There are no oostegites, the eggs being attached to the endopodites of the pleopods. The hepatic caeca are much ramified, the heart is abbreviated and saccular, the spermatozoa are spherical with radiating pseudopodia, and development is typically attended by a complicated larval metamorphosis.
Order I. Euphausiacea.

Fig. 101.—Calyptopis larva of Euphausia pellucida, × about 20. A.1, 1st antenna; Ab.6, 6th abdominal segment; E, eye; M, maxillipede. (After Sars.)
The Euphausiidae[116] agree with the Decapoda in passing through a complicated larval metamorphosis. The young hatch out as Nauplii, with uniramous first antennae and biramous second antennae and mandibles. In the next stage, or “Calyptopis” (Fig. 101), which corresponds exactly to the Zoaea of the Decapoda, two pairs of maxillae and a pair of biramous maxillipedes are added; the hinder thoracic segments are undifferentiated, but the abdomen is fully segmented, 145and the rudiments of the sixth pair of pleopods are already visible.
In the next stage (“Furcilia”) the other abdominal pleopods are added, the whole series being completed before the thoracic appendages number more than two or three. This stage corresponds to the Metazoaea of the Decapoda, and the interference in the orderly differentiation of the segments with their appendages from before backwards is a phenomenon which we shall meet again when we treat of Decapod metamorphosis. It is evidently a secondary modification, furnishing the larva precociously with its most important swimming organs so as to enable it to lead a pelagic existence. The frequent violation of the law of metameric segmentation, that the most anterior segments being the first formed should be the first to be fully differentiated, leads us to suppose that the larval stages of the Eucarida at any rate do not represent phylogenetic adult stages through which the Malacostraca have passed. Nor do they, perhaps, even represent primitive larval stages, but have been secondarily acquired from an embryonic condition which used to be passed through within the egg-membranes, as in Nebalia and the Mysidacea, when the order of differentiation of the segments was normal. The case is a little different with the Nauplius larva. This larval form, in an identical condition, is found both in the Entomostraca as a general rule, and again in certain Malacostraca, viz. the Euphausiidae and the Peneidea. Whatever its phylogenetic meaning may be, we may be quite certain that the ancestor of the two great divisions of the Crustacea had a free-swimming Nauplius larva, and this conclusion is confirmed by the probable presence of a Nauplius larva in Trilobites.
The Euphausiidae, in contradistinction to the Mysidae, are frequently met with in the surface-plankton. Euphausia pellucida (Fig. 102) is of universal distribution, and is frequently taken at the surface as well as at considerable depths.
Many noteworthy features in Euphausiid organisation are brought out in Fig. 102. The shrimp-like appearance of the carapace and antennae indicate the special Decapodan affinities of the family; noteworthy, also, are the single series of gills and the biramous thoracic and abdominal limbs, similar to those of the Mysidacea. The Euphausiidae also possess phosphorescent organs of a highly developed kind, and these are usually situated, 146as in the type figured, upon the outer margins of the stalked eyes, on the bases of the second and seventh thoracic limbs, and on the ventral median line on the first four abdominal segments. These organs are lantern-like structures provided with a lens, a reflector, and a light-producing tissue, and they are under the control of the nervous system. Their exact use is not known, any more than is the use of phosphorescence in the majority of organisms which produce it; but in certain cases it appears that the Euphausiids make use of their phosphorescent organs as bull’s eye lanterns for illuminating the dark regions into which they penetrate or in which some of them permanently dwell. At any rate, associated with the presence of these organs in some deep-sea Euphausiids are remarkable modifications of the eyes; and we may perhaps here fittingly introduce a short discussion of these visual modifications in deep-sea Crustacea, and the conditions which call them forth.

Fig. 102.—Euphausia pellucida, female, × 5. G, Last gill; L, luminous organ of first leg; L′, luminous organ of 2nd abdominal segment; T, biramous thoracic appendages. (After Sars.)

Fig. 103.—A, Sections (diagrammatic) of Crustacean compound eye, A, with pigment in light-position for mosaic vision; B, with pigment in dark-position for refractive vision. c, Corneal lens; c.g, corneagen cells; cr, crystalline cone; f, basal membrane, or membrana fenestrata; ip, irido-pigment; n, nerve; r, retinula; rh, rhabdom; rp, retino-pigment; v, vitrella.
The compound eyes of Crustacea resemble those of Insects in that they are composed of a very large number of similar elements or “ommatidia,” more or less isolated from one another by pigment. Each ommatidium consists typically of a corneal lens (Fig. 103, c), secreted by flat corneagen cells (c.g) below; beneath the corneal lens is a transparent refractive body called the “crystalline cone” (cr), which is produced by a number of cells surrounding it called the “vitrellae” (v). Below the crystalline cone comes the “rhabdom” (rh), produced and nourished by “retinulacells” 147(r). The rhabdom is a transversely striated rod, constituting the true sensory part of each ommatidium, and is in connexion at its lower end with a nerve-fibre (n), passing to the optic ganglion. The rhabdoms rest upon a membrane (f) called the “membrana fenestrata.” Each ommatidium is isolated from its fellows which surround it by a complete cylinder of pigment, part of which is especially crowded round the crystalline cone, and is known as “irido-pigment” (ip), while the part which surrounds the rhabdom is called “retino-pigment” (rp).
When the pigment is arranged in this way, as in Fig. A, only those rays of light which strike an ommatidium approximately at right angles to the corneal surface can be perceived, since only these can reach the top of the rhabdom; the others pass through the crystalline cones obliquely, and are absorbed by the cylinder of pigment surrounding each ommatidium, so that they neither reach the rhabdom of the ommatidium which they originally entered, nor can they penetrate to the rhabdom of neighbouring ommatidia. This gives rise to what is known as “mosaic vision,” that is to say, each ommatidium only perceives the rays of light which are parallel to its long axis, and in this way an image is built up of which the various points are perceived side by side by means of separate eye-elements. The distinctness and efficiency of this mode of vision depends chiefly upon the number of ommatidia present, and the completeness with which they are isolated from one another by the pigment. Now this form of vision, depending as it does 148upon the absorption of a great number of the light-rays by pigment, and the transmission of only a limited number to the sensory surface, is only possible when there is a strong light, and there is no need for economising the light-rays. The most important discovery was made by Exner,[117] that the majority of animals with compound eyes had the power of so arranging the pigment in their eyes as to enable them to see in two ways. In bright light the pigment is situated as in Fig. 103, A, so as completely to isolate the rhabdoms from one another (day-position); but in the dusk the pigment actively migrates, the irido-pigment passing to the surface (B) near the tops of the crystalline cones, and the retino-pigment passing interiorly to rest on the membrana fenestrata at the bases of the rhabdoms (night-position). When this happens the rays of light which strike the ommatidia at all sorts of angles, instead of being largely absorbed by the pigment, are refracted by the crystalline cones and distributed over the tops of the rhabdoms, passing freely from one ommatidium to another. In this way the eye acts on this occasion, not by mosaic vision, but on the principle of refraction, as in the Vertebrate eye. Of course the distinctness of vision is lost, but an immense economy in the use of light-rays is effected, and the creature can perceive objects and movements dimly in the dusk which by mosaic vision it could not see at all. The pigment is contained in living cells or chromatophores, and it is carried about by the active amoeboid movements of these cells with great rapidity.
Now, besides the active adaptability to different degrees of light brought about in the individual by these means, we find Crustacea living under special conditions in which the eyes are permanently modified for seeing in the dusk, and this naturally occurs in many deep-sea forms.
Doflein[118] has examined the eyes of a great number of deep-sea Brachyura dredged by the Valdivia Expedition, and as the result of this investigation he states that the eyes of deep-sea Brachyura are never composed of so many ommatidia, nor are they so deeply pigmented as those of littoral or shallow water forms. At the same time an immense range of variation occurs among deep-sea forms which are apparently subjected to similar 149conditions of darkness, a variation stretching from almost normal eyes to their complete degeneration and the fusion of the eye-stalks with the carapace; and this variation is very difficult to account for. A very frequent condition for crabs living at about 100 fathoms, and even more, is for either the irido-pigment or the retino-pigment to be absent, for the number of ommatidia to be reduced, and for the corneal lenses to be greatly arched. There can be little doubt that these crabs use their eyes, not for mosaic vision, but to obtain the superposition-image characteristic of the Vertebrate eye. In deeper waters, where no daylight penetrates at all, this type of eye is also met with, and also further stages in degeneration where all pigment is absent, and the ommatidia show further signs of reduction and degeneration, e.g. Cyclodorippe dromioides. In a few forms, e.g. Cymonomus granulatus among Brachyura, and numerous Macrura, the ommatidia may entirely disappear, and the eye-stalks may become fused with the carapace or converted into tactile organs.
Progressive stages in degeneration, correlated with the depth in which the animals are found, are afforded by closely related species, or even by individuals of apparently the same species. Thus in the large Serolidae of Antarctic seas, Serolis schytei occurs in 7–128 metres, and has well-developed eyes; S. bronleyana, from 730 to 3600 metres, has small and semi-degenerate eyes; while S. antarctica in 730–2920 metres is completely blind. Lispognathus thompsoni is a deep-water spider-crab, and the individuals taken at various depths are said to exhibit progressive stages in degeneration according to the depth from which they come.
At the same time many anomalies occur which are difficult to explain. In the middle depths, i.e. at about 100 fathoms, side by side with species which have semi-degenerate or, at any rate, poorly pigmented eyes, occur species with intensely pigmented eyes composed of very numerous ommatidia, e.g. the Galatheid Munidopsis and several shrimps, while in the true abysses many of the species have quite normal pigmented eyes. This is especially the case with the deep-sea Pagurids, of which Alcock describes only one species, Parapylocheles scorpio, as having poorly pigmented eyes. An attempt to account for this was made by Milne Edwards and Bouvier,[119] who pointed out that the truly deep-sea forms with well-developed eyes were always 150Crustacea of a roving habit, which were perhaps capable of penetrating into better lit regions, and to whom well-developed eyes might be useful, while the degenerate forms were sluggish. This explanation cannot be held to account for the phenomenon, as too many deep-sea forms with fairly normal eyes are known which are never taken outside deep waters. Doflein (loc. cit.) points out that in the Brachyura of the deep sea there is a remarkable correlation between the degree of degeneration of the eye and the size of the eggs—the large-egged forms having unpigmented and degenerate eyes, while the species with small eggs have pigmented eyes. He supposes that the species with large eggs undergo a direct development without pelagic free-swimming larvae, and that since they never reach the surface their eyes never meet with the necessary stimulus of light for the development of pigment; whereas the small-egged species undergo a pelagic larval existence when this stimulus is present and gives the necessary initiative for the development of the pigment.
Another factor enters into the question of eye-degeneration in the Crustacea. The great majority of deep-sea animals, including many deep-sea Crustacea, are phosphorescent, and it is certain that although daylight never penetrates into the abysses of the ocean, yet there is considerable illumination derived from the phosphorescence of the inhabitants of these regions.
Alcock[120] points out in this connexion that the Pagurids, which are conspicuous in the great depths as animals with normally developed eyes, carry about anemones with them, and these organisms are very frequently phosphorescent to a high degree. It may well be, therefore, that the Pagurids are enabled to use their eyes in the normal manner owing to the phosphorescent light which they carry about with them, and this use of phosphorescent light may apply to a number of deep-sea Crustacea whose eyes are not at all or only partially degenerate.
An extremely interesting case of the use of phosphorescent light is given by Chun.[121] In a number of Euphausiids occurring in deep waters each compound eye is divided into two parts—a frontal and ventro-lateral—which differ from one another very greatly in the nature and disposition of their ommatidia.

Fig. 104.—Section of eye of Stylocheiron mastigophorum. A, Frontal portion; B, ventro-lateral portion; C, phosphorescent organ; D, entrance of optic nerve; c, corneal lens; cr, crystalline cone; pg, pigment; ret, retinula; rh, rhabdom. (After Chun.)
In the frontal portion (Fig. 104, A) the ommatidia are few in 151number and long, the corneal lenses are highly arched, and the pigment is reduced to a few clumps in the iris. This part of the eye is evidently adapted for forming a vague superposition-image in the dusk. The ventro-lateral part (B), on the other hand, is composed of numerous small ommatidia, the crystalline cones of which can be completely isolated from one another by the irido-pigment. Immediately below this part of the eye is a phosphorescent organ (C) provided with a lens and tapetum. Chun suggests that the ventro-lateral part of the eye is used for obtaining a clear mosaic image of objects illuminated by the phosphorescent organ, while the frontal part of the eye is used for obtaining general visual impressions in dimly lit regions. This curious differentiation of the eye into two parts apparently only occurs in predaceous animals, which capture their prey alive upon the bottom, and to whom a clear vision of moving organisms is a necessity.
Another instance of Crustaceans making use of their own light is given by Alcock,[122] who found two deep-sea prawns, Heterocarpus alphonsi and Aristaeus coruscans, at about 500 fathoms in the Indian Ocean. These animals produce a highly phosphorescent substance which they eject from the antennary glands, and they possess very large, deeply-pigmented eyes.
The whole subject of the modification of the pigment and structure of Crustacean eyes is an interesting one, because it presents us with one of those cases in which the direct response to a stimulus acting within the lifetime of the individual seems to run parallel to the fixed adaptations of a whole species, which have become hereditary and apparently independent of the external stimulus of light or of the absence of light. As far 152as is known, however, the direct response of the individual to the absence of light is limited to the reduction or disappearance of the pigment, and does not extend to those structural changes in the ommatidia which are characteristic of so many deep-sea forms.
Order II. Decapoda.[123]
The Decapoda, together with the Euphausiidae, make up the Division Eucarida, the members of which differ from the Orders hitherto described in a number of characters, e.g. the presence of a carapace covering the whole of the thorax, the absence of a brood-pouch formed of oostegites, the presence of a short heart, of spermatozoa with radiating pseudopodia, and of a complicated larval metamorphosis, of which the Zoaea stages are most prominent.
The Decapoda differ from the Euphausiidae chiefly in the anterior three thoracic limbs being turned forwards towards the mouth to act as maxillipedes, and in the five succeeding thoracic limbs being nearly always uniramous and ambulatory or chelate; there are typically present three serial rows of gills attached to the thoracic segments, an upper series (“pleurobranchiae”) attached to the body-wall above the articulation of the limbs, a middle series (“arthrobranchiae”) attached at the articulation of the limbs, and a lower series (“podobranchiae”) attached to the basal joints of the limbs. These gills are enclosed in a special branchial chamber on each side of the thorax, formed by lateral wings of the carapace known as “branchiostegites.” The gills of each series are never all present in the same animal, the anterior and posterior members showing a special tendency to be reduced and to disappear. In this manner “branchial formulae” can be constructed for the various kinds of Decapods, which differ from the ideal formula in a manner distinctive of each kind. The second maxilla is always provided with an oar-like appendage on its outer margin (exopodite), known as the “scaphognathite,” which, by its rhythmical movement, keeps up a constant current of water through the gill-chamber.
A complicated auditory organ is present on the basal joint of the first antennae; this is a sac communicating with the exterior and lined internally with sensory hairs. The animal is 153said to place small pieces of sand, etc., in its ears to act as otoliths. Anaspides (see p. 116) is the only other Crustacean which has an auditory organ in this position.
The larval histories of the Decapods[124] are of great interest, and will be given under the headings of the various groups. The first discoverer of the metamorphosis of the Decapoda was the Irish naturalist J. V. Thompson, certainly one of the ablest of British zoologists. In 1828, in his Zoological Researches, he describes certain Zoaeas of the Brachyura and proves that these animals are not an adult genus, as supposed, but larval forms. But Rathke, in 1829, described the direct development of the Crayfish; and Westwood, after describing the direct development of Gecarcinus, utterly denied Thompson’s assertions concerning metamorphosis. Thompson replied in the Royal Society Transactions for 1835, and described the Megalopa stage of Cancer pagurus. Rathke,[125] although previously an opponent of Thompson, subsequently made confirmatory observations upon the larvae of the Anomura; and Spence Bate clinched the matter by describing Brachyuran metamorphosis with great accuracy in the Philosophical Transactions for 1859. Since then a mass of work has been done on the subject, though much detail still remains to be elucidated.
The Decapoda fall into three sub-orders, which graduate into one another—(i.) the Macrura, including the Lobsters, Crayfishes, Shrimps, and Prawns; (ii.) the Anomura, including the Hermit-lobsters and Hermit-crabs; and (iii.) the Brachyura or true Crabs.
Sub-Order 1. Macrura.
This sub-order[126] is characterised by the large abdomen, furnished with five pairs of biramous pleopods, and ending in a powerful tail-fan composed of the telson and the greatly expanded sixth pair of pleopods, the whole apparatus being locomotory. The second antennae are furnished with very large external scales, representing the exopodites of those appendages. Some of the Shrimps and Prawns closely resemble the “Schizopods,” but the pereiopods are nearly always uniramous.[127] Several subdivisions of the Macrura are recognised.
Tribe 1. Nephropsidea.
This tribe includes the Lobsters and Crayfishes, animals well known from their serviceableness to man. There are three families, which will be treated separately.
Fam. 1. Nephropsidae. The podobranchs are not united with the epipodites, and the last thoracic segment is fixed and fused to the carapace. The chelae are generally asymmetrical. The most important Lobsters are the European and the American species—Homarus vulgaris (= Astacus gammarus) and H. americanus respectively; these animals engage a large number of people in the fisheries. It is estimated that in America about £150,000 are spent every year on Lobsters.
The genus Nephrops contains the small Norwegian lobster and other forms.
Herrick[128] gives some interesting particulars with regard to the life-history of the American species. The largest recorded specimen weighed about twenty-five pounds, and measured twenty inches from rostrum to tail; similar European specimens have been recorded, but, on the average, they are not so large as the American forms.
The Lobster, like all Crustacea, undergoes a series of moults as the result of increase in size, shedding the whole of the external integument in one piece. This is accomplished by a split taking place on the dorsal surface at the junction of thorax and abdomen; through the slit so formed the Lobster retracts first his thorax with all the limbs, and then his abdomen. When first issuing from the old shell the animal’s integument is soft and pulpy, but the increase in size of the body is already manifest; this increase per moult, which is approximately the same in young and adult animals, varies from 13 to 15 per cent of the animal’s length. According to this computation, a Lobster 2 inches long has moulted fourteen times, 5 inches twenty times, and 10 inches twenty-five times, and it may be roughly estimated that a 10–inch Lobster is four years old. Young Lobsters probably moult twice a year, and so do adult males, but females only moult once a year soon after the young are hatched out.
The process of moulting or ecdysis is an exceedingly 155dangerous one to the Lobster and to Crustacea in general, and is very frequently fatal. There is, first of all, the danger of the act not being accomplished skilfully, when death always ensues. The Lobster remains soft and unprotected for about six weeks after the ecdysis, and is very apt to fall a prey to the predaceous fish, such as Sharks, Skates, Cod, etc., which feed upon it. There are, however, some peculiar adaptations connected with the process which are of interest. In order to facilitate the ecdysis, areas of absorption are formed upon the dorsal and ventral surfaces of the carapace, on the narrower parts of the chelipedes, and at other places; in these areas the calcium carbonate is absorbed, and the old shell becomes elastic and thin, so as to allow a more easy escape for the moulting Lobster. It has been noticed that while this is taking place large concretions of calcium carbonate are formed at the sides of the stomach, known as “gastroliths,” which perhaps represent the waste lime that has been abstracted from the areas of absorption. After moulting the Lobster is in great need of lime for stiffening his shell, and it has been noticed that on these occasions he is very greedy of this substance, even devouring his own cast-off skin.
The male Lobster is especially prized on account of his larger chelae, but in both sexes the chelipedes are differentiated into a smaller cutting pincer and a larger crushing one. Lobsters may be right or left handed, with the large crushing claw on the right or left hand, and sometimes specimens occur with the smaller cutting pincers on both chelipedes, and very rarely, indeed, with crushing claws on both sides. Crustacea very commonly have the power of casting off a limb if they are seized by it or if it is injured, and of regenerating a new one. In the Lobster a so-called breaking joint is situated on each leg at the suture between the fused second and third segments; a membrane being pushed inwards from the skin, which not only serves to form a weak joint where rupture may easily take place, but also to stop excessive bleeding after rupture. In the newly-hatched larvae there is a normal joint between the second and third segments; and autotomy, or the voluntary throwing away of a limb, never occurs until the fourth larval stage, when the breaking joint is formed. Autotomy is a reflex act under the control of the segmental ganglion; if a Crab or Lobster be anæsthetised, and then a limb be injured or broken off below the 156breaking joint, the animal forgets to throw the injured leg or stump off at the breaking joint, a proceeding which always occurs under normal conditions. The regeneration of a limb starts from a papilla which grows out of the breaking joint, and after a number of moults acquires the specific form of the limb that has been lost. A number of interesting observations have been made upon the regeneration of the limbs in Crustacea. It was in the Hermit-crab that Morgan[129] proved that regeneration and the liability to injury do not always run parallel, as Weismann held they should, since the rudimentary posterior thoracic limbs, which are never injured in nature, can regenerate when artificially removed as easily as any others. Przibram[130] has shown that in the shrimp Alpheus, whose chelipedes are highly asymmetrical, if the large one be cut off, the small one immediately begins to grow and to take on the form of the large one, while the regenerated limb is formed as the small variety. This remarkable inversion in the symmetry of the animal clearly ensures that, if the large chela is injured and thrown away, the least amount of time is wasted in providing the shrimp with a new large claw.
To return to the Lobster; for the majority of the individuals there is a definite breeding season, viz. July and August, but a certain proportion breed earlier or later. A female begins to “berry” at about eight inches in length, and to produce more and more eggs up to about eighteen inches, when as many as 160,000 eggs are produced at a time; after this there is a decline in numbers. A female normally breeds only once in two years. Strict laws are enforced forbidding the sale of Lobsters and Crabs “in berry” in both England and America. The period of incubation, during which the developing eggs are attached to the swimmerets of the female, lasts about ten or eleven months, so that the larvae are hatched out approximately in the following June. On hatching, the larva, which measures about one-third of an inch, and is in the Mysis stage (i.e. it possesses all the thoracic limbs in a biramous condition, but is without the abdominal limbs), swims at first on the surface. After five or six months of this life, during which the abdominal pleopods are added from before backwards, it sinks to the bottom, loses the 157exopodites of the thoracic limbs, and is converted into the young Lobster, measuring about half an inch in length. The little Lobster starts in deepish water, and gradually crawls towards the shore; here it passes its adolescence, but on coming to maturity it migrates out again into the deep water.
Fam. 2. Astacidae.—In this family, which includes all the European and North American Crayfishes, Astacus (Potamobius) and Cambarus, the podobranchs are united with the epipodites, the last thoracic segment is free, there is only one pleurobranch or none at all, the gills have a central lamina, but the filaments are without terminal hooks, and the endopodites of the first two pairs of abdominal appendages in the male serve as copulatory organs. For the distribution, etc., of these forms see p. 213.
Fam. 3. Parastacidae.—This family includes the Crayfishes of the Southern Hemisphere, viz. Parastacus from South America, Astacopsis and Engaeus from Australia, Paranephrops from New Zealand, and Astacoides from Madagascar. These genera agree with the Potamobiidae in the union of the podobranchs with the epipodites, and in the free condition of the last thoracic segment, but there are generally four pleurobranchs, the gills are without a lamina, the filaments have terminal hooks, and there are no sexual appendages in the male. For distribution, etc., see also p. 213.
The larval development in the Crayfishes is still more abbreviated than in the Lobsters, the Mysis stage being passed through within the egg-membranes. The young, when they hatch out, are furnished with hooks upon the chelipedes, by which they anchor themselves to the pleopods of the mother.
Tribe 2. Eryonidea.

Fig. 105.—Willemoesia inornata, × ⅓. (From a figure prepared for Professor Weldon.)
These are remarkably archaic animals of great rarity, though they were common enough in Triassic seas, and have come down to us as fossils from those times, being thus among the oldest Decapoda known. They only survive now as deep sea species, and the genus discovered by the Challenger,[131] Willemoesia (Fig. 105), confirmed the expectations of the Challenger naturalists that the abysses of the ocean would contain relics from older periods which 158had managed to survive where the competition was not so keen. The genus Willemoesia is very widely distributed, being dredged up from below a thousand fathoms in the Indian Ocean, the Mediterranean, North and South Atlantic, and the Pacific oceans. All the walking legs are chelate, and the animal is quite blind, as are all the Eryonidea, the eye-stalks being fused with the carapace.
Only a single family Eryonidae is recognised.
Tribe 3. Peneidea.—Tribe 4. Caridea.
We will now consider the Shrimps and Prawns, since in them occurs the most complete metamorphosis found in the Decapoda. The Peneidea are distinguished from the ordinary Prawns and Shrimps (Caridea) by having the first three instead of the first two pereiopods chelate. The genus Peneus affords several species which are of commercial value as objects of food; the edible Prawns of the Mediterranean belong to this genus, while in the North Sea two of the Caridea, viz. the Shrimp, Crangon vulgaris, and the Prawn, Palaemon serratus, are the forms very commonly eaten. Both subdivisions are well represented in the deep sea fauna from all parts of the world. Glyphocrangon spinulosa (Fig. 110, p. 164) is a deep sea Shrimp with eyes that have lost their pigment, and with the body covered with spines, while the last abdominal segment is fused with the telson to form a sharp bayonet-like process at the hind end of the body. Some of the deep-sea Prawns of the Indian Ocean 159are described by Alcock[132] as possessing peculiar secondary sexual characters. Thus Parapeneus rectacutus ♂ has one lash of the first pair of antennae peculiarly bent to form a clasping organ, while Aristaeus crassipes has a hook on the end of the third maxillipede. In the latter the females have much longer rostra than the males, and are in general more powerfully built, so that they seem to have usurped the proper functions of the male, and probably engage in combats with one another over his person.

Fig. 106.—Nauplius larva of Peneus, sp. × 25. (From Balfour, after F. Müller).
As a general rule the Shrimps and Prawns occur in large shoals in the shallow waters of the littoral zone, and they have a remarkable power of adapting their colours to the surroundings in which they happen to be at any particular moment.[133] This is brought about by the variously coloured chromatophores, which contract and expand in obedience to a stimulus transmitted through the eyes of the animal. A number of the Palaemonidae go up rivers into fresh water, while one family, the Atyidae, live in the completely fresh water of rivers and inland lakes. The Peneidea undergo a very complete metamorphosis which is primitive in respect to the order of formation of the segments from before backwards. The larva hatches out as a Nauplius (Fig. 106), which by the orderly addition of segments 160behind is converted into the Protozoaea (Fig. 107), possessing two pairs of biramous maxillipedes. It should be noted that the maxillae, which are foliaceous in the adult, are laid down in this condition in the larva, and this principle holds good throughout Crustacean metamorphosis, viz. that when a limb is foliaceous in the adult it is foliaceous in the larva, and when biramous in the adult it is biramous in the larva. Whilst the rest of the thoracic limbs are still rudimentary, the sixth pair of pleopods are being precociously developed (Fig. 108), being the only precociously formed limbs in the Peneidea, though the abdominal segments are fully marked off before the thoracic segments, and so must be considered as precocious in development. When the biramous thoracic limbs are completed the abdominal biramous pleopods are added, beginning from in front backwards. Thus the Mysis stage (Fig. 109) is reached, which resembles in all particulars the adult condition of the Schizopoda. The adult Prawn develops from this stage by the loss of some or all of the exopodites on the thoracic pereiopods.

Fig. 107.—Protozoea larva of Peneus, sp. × 25. (From Balfour, after F. Müller.)

Fig. 108.—Zoaea larva of Peneus, sp. × 25. A, A′, 1st and 2nd antennae; Ab.6, 6th abdominal appendage; Mxp, 2nd maxillipede; T, 4th–8th thoracic appendages (future walking legs). (After F. Müller.)
161Some of the Peneid larvae take on very peculiar forms, e.g. the Zoaeae of the Sergestidae,[134] which often develop the most wonderful spines all over the body.

Fig. 109.—Mysis stage in the development of Peneus, sp. A.2, 2nd antenna; Ab.6, 6th abdominal appendage; T, telson; Th, the biramous thoracic appendages. (After Claus.)
The Caridea have a greatly abbreviated metamorphosis, the larva hatching out at a late Zoaea stage with all three pairs of maxillipedes fully formed and with a fully segmented abdomen. The succeeding thoracic limbs are added in order from before backwards, though the sixth pair of pleopods appear precociously as in the Peneidea. The other swimmerets do not begin to develop until the thoracic limbs are complete. Some Caridea show a yet more abbreviated metamorphosis, e.g. the fresh-water Palaemonetes varians of S. Europe, which hatches out at the Mysis stage.
We see, therefore, in the metamorphosis of the Macrura several apparently primitive features. In the first place, a free swimming Nauplius stage is preserved in certain forms, identical 162in all respects with the Nauplius of the Entomostraca. Secondly, the thoracic limbs when they are first developed are biramous, thus giving rise to the characteristic Mysis stage which links the Macrura on to the “Schizopoda.” Thirdly, the order of differentiation of the segments is typically from in front backwards, the only precociously developed appendage being the sixth abdominal. None of these characters are reproduced in the higher Decapoda in which there is never a free-living Nauplius, the first larval stage being the Zoaea; a number of the thoracic pereiopods, and usually all of them, are uniramous from the start; and the whole of the abdominal segments with their limbs tend to be precociously developed before the hinder thoracic segments make a distinct appearance.
Tribe 3. Peneidea.[135]
The third legs are chelate except in genera in which the legs are much reduced. The third maxillipedes are seven-jointed, the second maxillipedes have normal end-joints, and the first maxillipedes are without a lobe on the base of the exopodite. The pleura of the first abdominal segment are not overlapped by those of the second. The abdomen is without a sharp bend. The branchiae are usually not phyllobranchs.
Fam 1. Peneidae.—The last two pairs of legs are well developed, and there is a nearly complete series of gills. Cerataspis,[136] a pelagic form. Parapeneus, Peneus, Aristaeus, etc.
Fam. 2. Sergestidae.—The last or last two pairs of legs are reduced or lost. The gill-series is incomplete or wanting. Sergestes possesses gills, and the front end of the thorax is not greatly elongated. Lucifer has no gills, and the front of the thorax is greatly elongated, giving a very anomalous appearance to the animal. All the members of this family are pelagic in habit.
Fam. 3. Stenopodidae.—One or both legs of the third pair are longer and much stouter than those of the first two pairs. On a number of small anatomical points this family, including the littoral genus Stenopus from the Mediterranean and other warmer seas and Spongicola commensal with Hexactinellid sponges from Japan, is separated by some authors in a Tribe by itself.
Tribe 4. Caridea.
The third legs are not chelate. The third maxillipedes are 4–6 jointed, the end-joint of the second maxillipede nearly always lies as a strip along the end of the joint before it, and the first maxillipedes have a lobe on the base of the exopodites. The pleura of the second abdominal segment overlap those of the first. The abdomen has a sharp bend; the branchiae are phyllobranchs.
Fam. 1. Pasiphaeidae.—In this family the end-joint of the second maxillipedes is normally formed, and exopodites are usually present on all the thoracic limbs. Rostrum small or wanting. Rather numerous genera are known, most of which inhabit the deep sea, though a few come into the littoral zone. Pasiphaea chiefly in the deep sea, Leptochela in the tropical littoral zone.
Fam. 2. Acanthephyridae.—The end-joint of the second maxillipede is modified as in other Caridea, and the rostrum is very strong and serrate, but in the presence of exopodites, and in the form of the mouth-parts, this family agrees with the preceding. It is also a characteristic deep-sea family. Acanthephyra, Hymenodora, Nematocarcinus, etc.
Fam. 3. Atyidae.—This is an entirely fresh-water family, especially characteristic of the rivers and lakes of the tropics, some of the forms being exceedingly large and taking the place of the Crayfishes in these waters. Characteristic of this family is the fact that the fingers of the chelae are spoon-shaped, and carry peculiar tufts of bristles. Exopodites are present on the thoracic limbs of some of the genera (Troglocaris, Xiphocaris from Australia and the Malay Islands, Atyephyra from S. and W. Europe), but are absent in others. Caridina, widely spread and common in Indo-Malay and Africa; Atya from West Indies, West Africa, and Pacific Islands.
Fam. 4. Alpheidae.[137]—The exopodites are absent, and the rostrum is absent or very feeble. The chelae are powerful, and usually very asymmetrically developed. Alpheus has an enormous number of species which live chiefly in the tropical seas, where they haunt especially the coral-reefs, making their homes among the coral or in sponges, etc. Although occurring in the Mediterranean they penetrate very rarely into colder seas.
164Fam. 5 Psalidopodidae.—This family, characterised by the absence of chelae on the second thoracic limbs, which carry instead a terminal brush of hairs, and by the rudimentary condition of the eyes, is represented by the genus Psalidopus from the deep waters of the Indian Ocean.
Fam. 6. Pandalidae.—The first thoracic limb is without chelae, only six-jointed. The rostrum is large and toothed. The genus Pandalus has numerous representatives in the northern littoral, P. annulicornis being one of the prawns most commonly met with in the fish-markets.
Fam. 7. Hippolytidae.—The first and second thoracic limbs bear chelae, the carpus of the second being divided into two or more segments. The first pair of chelae are not distinctly stronger than the second. Virbius has many species in the littoral zone of all seas, and one species, V. acuminatus, is pelagic. Hippolyte also has numerous littoral forms distributed all over the world, but chiefly in the arctic or subarctic seas. H. varians, common on the English coasts, shows interesting colour-reactions to its surroundings.[138]

Fig. 110.—Glyphocrangon spinulosa, from the right side, × 1. (From an original drawing prepared for Professor Weldon.)
Fam. 8. Palaemonidae.—The first two pairs of legs are chelate, the carpus of the second not being subdivided. Palaemon serratus, a very common prawn in the British littoral. Palaemonetes in the brackish and fresh waters of Europe and N. America.
Fam. 9. Glyphocrangonidae.—The first pair of legs are subchelate, the carpus of the second pair is subdivided, and the rostrum is long. Glyphocrangon (Fig. 110) with numerous species entirely confined to deep water.
Fam. 10. Crangonidae.—The first pair of legs are subchelate, the carpus of the second pair is not subdivided, and the rostrum is short. Crangon vulgaris is the common Shrimp of the North Sea.
Tribe 5. Loricata.

Fig. 111.—Dorsal view of Scyllarus arctus, × ½. (From an original figure prepared for Professor Weldon.)

Fig. 112.—Embryonic area of developing Palinurus quadricornis. Ab.1, 1st abdominal segment; E, compound eye; E′, median simple eye; L, upper lip; L′, lower lip; M, mandible; Mx.1, Mx.2, 1st and 2nd maxillae; Mxp.1, 1st maxillipede; T, 6th (antepenultimate) thoracic appendage. (After Claus.)
The Loricata include the Langouste (Palinurus) of the Mediterranean coasts, which replaces there the Lobster of the North Sea as an article of food, and the peculiarly shaped Scyllarus arctus (Fig. 111), which is also prized in the Mediterranean as a delicacy. The bright red “Crayfishes,” Panulirus and Iasus, of the Australian coasts are also largely used as food. Besides its peculiarity in shape, S. arctus has remarkable scales on the second antennae in place of flagella. The larva hatches out as the so-called Phyllosoma, 166which must be regarded as a greatly flattened and modified[139] Mysis stage.

Fig. 113.—Phyllosoma larva of Palinurus, sp. × 5. Ab, Abdomen; Mxp, 3rd maxillipede; T, antepenultimate (6th) thoracic appendage. (After Claus.)
In the embryo of Palinurus just before hatching (Fig. 112) we can recognise the limbs of the head and thorax normally developed in order. There are present three thoracic limbs, besides the maxillipedes. When the Phyllosoma hatches out the first maxillipedes have become quite rudimentary, and the second much reduced, while the second antennae and second maxillae are also reduced in size. The metamorphosis is completed by the re-development of the limbs and segments that have been secondarily suppressed during larval life, and by the appearance of the pleopods.
This process is again met with in the Squillidae (p. 143), but it resembles the suppression, in so many Decapodan metamorphoses, of anterior limbs and the precocious development of segments and limbs lying posteriorly. In the ordinary Decapoda, however, the suppressed limbs are merely not formed till later; while in the Loricata the limbs develop in the correct order, and subsequently degenerate. It is natural to wonder whether the condition of affairs in the Loricata represents the primitive process, and whether the precocious development of segments in the other Decapoda owes its origin to these animals having once had the direct mode of development when the segments were formed in the proper order, and to their having subsequently acquired the larval stages first of all by the degeneration, and then by the suppression of certain segments which were not of use during larval life. The complete metamorphosis, however, of the Peneidea, in which the segments and limbs appear in the 167right order, rather goes to show that this is the primitive mode of development in the Decapoda, and that the disarrangement in the order of appearance of the segments, both in the Squillidae and in the Loricata and other Decapods, has been independently acquired in the two cases to meet the needs of the larval existence.
Fam. 1. Palinuridae.—The cephalothorax is subcylindrical, the eyes are not enclosed in separate orbits formed by the edge of the carapace, and the second antennae possess flagella. Palinurus, with P. elephas, the European Rock Lobster or Langouste. Iasus with two species in the Antarctic littoral; Panulirus in the tropical littoral.
Fam. 2. Scyllaridae.—The cephalothorax is depressed, the eyes are enclosed in separate orbits formed by the edge of the carapace, and the second antennae have flat scales in the place of flagella. Scyllarus (Fig. 111), with the European S. arctus; Ibacus in rather deep water with several species, chiefly found in the southern hemisphere.
Tribe 6. Thalassinidea.
This tribe is included by some authors in the Anomura, and held to be closely related to the Galatheidea, but the unreduced abdomen is carried straight and unflexed, and gives a very Macrurous appearance to the animal. The Anomurous characters are the frequent reduction or absence of the antennal scale, the fact that only the first two pairs of pereiopods are ever chelate, and the reduced series of gills. The body is symmetrical, but the first pair of chelae is always highly asymmetrical. The posterior pairs of pereiopods, although small, are not characteristically reduced as in the Anomura. The animals belonging to this Tribe attain two or three inches in length, and generally burrow in sand or mud either in the littoral zone or in deeper waters; at the same time they can swim with considerable activity by means of the pleopods.
Fam. Callianassidae.—Callianassa subterranea is common at Naples, Gebia littoralis in the North Sea.
Sub-Order 2. Anomura
In this division are included the so-called Hermit-lobsters and Hermit-crabs, in which the condition of the abdomen is roughly intermediate between that of the Macrura and that of the Brachyura. 168It is not much reduced in size, and the pleopods of the sixth pair are fairly well developed, but it is usually carried flexed towards the thorax, and is never a powerful locomotory organ as in the Macrura. The antennal scale, if present at all, is a mere spine, not the large leaf-like structure of the Macrura; and there is never a partition between the two first antennae as in the Brachyura.
The last or last two pairs of pereiopods are reduced, and are turned on to the dorsal surface or carried inside the branchial chamber; but this curious character is met with again in certain Brachyura (Dromiacea and Oxystomata).
Tribe 1. Galatheidea.[140]

Fig. 114.—Dorsal view of Munidopsis hamata, × ½. (From an original figure prepared for Professor Weldon.)
These are symmetrical crabs with a long carapace; the abdomen, which is as broad as the carapace, is always carried flexed under the thorax, and the sixth pair of pleopods are expanded to form with the telson a fan-like tail. The most anterior pereiopods are always much elongated and chelate; while the last pair are much reduced, and either turned up on to the dorsal surface, or else carried in the branchial chamber. The exact meaning of this last characteristic in these forms is doubtful; some of the species are said to carry shells temporarily upon their backs, a proceeding probably assisted by the last pair of thoracic limbs, while in others their limbs may be used for cleaning out the branchial chamber. Most of the Galatheidea, for instance, the common Porcellana and 169Galathea, are littoral animals, and may be found hiding under stones and in crevices on the shore; but a number occur in deep water, e.g. Munida and Munidopsis.

Fig. 115.—Zoaea of Porcellana, × 20. T, Telson. (After Claus.)
The shallow-water species have ordinarily developed eyes; the various species of Munida, which occur in fairly deep but by no means abyssal regions, have usually very large and highly pigmented eyes; while in Munidopsis, which is characteristic of very deep water, the eyes are degenerate and colourless, as shown in Fig. 114.
The Zoaeae, or young larval stages of the Galatheidea, are characterised by the immense length of the spines upon the carapace (Fig. 115). The young Zoaea which hatches out from the egg resembles in other respects that of the Brachyura. The Metazoaea, however, differs from that of the Brachyura in the fact that the third maxillipede is first present as a biramous swimming organ, and at its first appearance is not developed in its definitive form. The other thoracic limbs are not schizopodous when they appear, and indeed in nearly all respects the development proceeds as in the Brachyura.
Fam. 1. Aegleidae.—The gills are trichobranchiae, and there are eight arthrobranchs. There are no limbs on the second abdominal segment of the male. The abdomen is not carried folded on to the thorax. The first two characteristics separate this family from all the other Galatheidea. Aeglea laevis, a fresh-water species from the rivers of temperate S. America, is the sole representative.
Fam. 2. Galatheidae.—The abdomen is not folded against the thorax. The members of this family are often littoral in 170habit (Galathea, Fig. 116), but often go down into great depths (Munidopsis, Fig. 114).

Fig. 116.—Dorsal view of Galathea strigosa, × ½. (From an original figure prepared for Professor Weldon.)
Fam. 3. Porcellanidae.—The abdomen is folded against the thorax, and the body has a crab-like form. These are always littoral in habit, never descending into the depths. Pachycheles in the tropics, Porcellana with numerous species in all seas, P. platycheles being a common British species.
Tribe 2. Hippidea.
The Mole-crabs have the habit of burrowing in sand, and their limbs are peculiarly modified into digging organs for this purpose (see Fig. 117). In other respects they are seen to be closely related to the Galatheidea by the form of the carapace, the condition of the abdomen, and the reduced last thoracic limbs.
In Albunea, which is found in the Mediterranean, the first antennae[141] are greatly lengthened and apposed to one another, and by means of a system of interlocking hairs they form a tube down which the water is sucked for respiration. The object of this arrangement is to ensure a supply of clear water, filtered from particles of sand, when the crab is buried beneath the surface, on these occasions the tip of the antennal tube being protruded above the surface of the sand. An exactly similar tube is used by the true Crab Corystes cassivelaunus, which has similar burrowing habits, but here the tube is formed from the second antennae and not from the first, so that the tubes in the two cases afford beautiful instances of analogous or homoplastic structures between which there is no homology (see p. 189).
171Fam. 1. Albuneidae.—The first legs are subchelate; the carapace is flattened, without expansions covering the legs. Albunea with several species in the Mediterranean, West Indies, and Indo-Pacific.

Fig. 117.—Remipes scutellatus, dorsal and ventral views, × 1. (From original drawings prepared for Professor Weldon.)
Fam. 2. Hippidae.—The first legs are simple, the carapace is subcylindrical with expansions covering the legs. Remipes (Fig. 117) and Hippa in tropical or sub-tropical seas.
Tribe 3. Paguridea.[142]
The ordinary Hermit-crabs, common on the English as on every coast, are characterised by the fleshy asymmetrical abdomen from which all the hard matter has disappeared, and which is carried tucked away in an empty Gasteropod shell. The abdomen is spirally wound in accordance with the shape of the shell, and a firm attachment is effected by means of the sixth pair of pleopods, especially that of the left side, which is fashioned into the form of a hook and is curled round the columella of the shell; this attachment is so secure that in trying to pull a Hermit-crab out of its shell the body is torn apart before the hold gives way. The other pleopods are in a much reduced condition, being generally altogether absent from the right side of the abdomen, 172and often greatly reduced on the left side, especially in the male, though in the female they are still used for the attachment of the eggs.
The last two pereiopods are much reduced and are concealed inside the shell, which they help to carry. The great chelae are usually asymmetrically developed, that on the right side being much larger than that on the left, and often serving the purpose of shutting the entrance to the shell when the crab is withdrawn inside.
The constant association of a large group of animals like the Hermit-crabs with the appropriated empty houses of another group is sufficiently curious, but it does not stop there. In almost every case there are present one or more Sea-anemones growing on the outside of the shell, and each kind of Hermit-crab generally carries a special kind of Anemone. Thus at Plymouth, Eupagurus bernhardus is generally symbiotic with Sagartia parasitica, or else with a colony of Hydractinia echinata, while E. prideauxii is usually associated with Adamsia palliata. In the latter case the shell is frequently absorbed, so that the Anemone comes to envelop the crab like a blanket. Instead of Anemones carried turret-like and imposing aloft, or enveloping the inmate of the shell like a blanket, some of the Hermits have Sponges, an unexpected association; and it is a common sight at Naples to find the little red round Sponge, Suberites, running around animated by its Hermit within. It is held that Anemone and crab mutually assist one another, that the Anemone stings the crab’s enemies, and that the Hermit-crab carries the Anemone to new feeding-grounds. It is also said that when a crab grows too big for its shell, and is forced to seek another, it persuades the Anemone to loosen its attachment to the deserted shell and to be transplanted to the new one, and that there is something mesmeric in its power, because nobody else can pull an Anemone off a shell without either cutting it off at the base or tearing it to pieces. Other animals as well sometimes enter into this partnership. At Plymouth a Polychaet worm, Nereis fucata, frequently inhabits the Whelk’s shell, together with Eupagurus bernhardus, and puts out its head for a share of each meal; and at Naples the Amphipod Lysianax punctatus is almost always present in the shells of Eupagurus prideauxii.

Fig. 118.—Pylocheles miersii, × 1. A, End view of a piece of mangrove or bamboo, the opening of which is closed by the great chelae (c) of the Pagurid; B, the animal removed from its house. (After Alcock.)
Besides the ordinary twisted Pagurids which inhabit Gasteropod shells, there are a few which preserve the symmetry of the body. The interesting Pylocheles miersii[143] (Fig. 118), taken by the Investigator in the Andaman Sea at 185 fathoms, inhabits pieces of bamboo; it is perfectly symmetrical, with well-developed pleopods and symmetrical chelae, which, when the animal is withdrawn, completely shut up the entrance to its house (Fig. 118, A).
It is doubtful whether this animal ever inhabited a spiral shell or not in its past history; but there is no doubt that a number of peculiar crabs, which caused the older systematists much trouble, are Pagurids, derived from asymmetrical shell-haunting ancestors that have secondarily taken to a different mode of life, and lost, or partially lost those characteristics of ordinary Hermit-crabs which are associated with life in a spiral shell. These are the Lithodidae and the “Robber-crab,” Birgus latro, of tropical coral islands.
Although the Robber-crab and the Lithodidae bear a certain superficial resemblance to one another in that they lead a free existence, and have reacquired to a great extent their symmetry, yet it is clear that they have been independently derived from different groups of asymmetrical Hermit-crabs, and that their resemblance to one another is due to convergence.
Birgus latro (Fig. 119), a gigantic crab, frequently over a foot in length, lives on land, and inhabits the coasts of coral islands in the Indian and Pacific Oceans where cocoa-nut trees 174grow. It feeds on the pulp of the cocoa-nut, which it extracts by hammering with its heavy chela on the “eye-hole” until room is made for the small chela to enter and extract the pulp. There is not the slightest doubt that the animal often ascends the cocoa-nut trees for the purpose of picking the nuts, a fact illustrated by a fine photograph by Dr. Andrews, exhibited in the Crustacean Gallery in the Natural History Departments of the British Museum. It uses the husk of the nut to line its burrow, and it is said to have the habit of putting its abdomen into the nut-shell for protection and carrying it about with it. Owing to its terrestrial mode of life, the branchial chamber is highly modified, being divided into two portions—a dorsal space, the lining of which is thrown into vascular ridges and folds for aerial respiration, and a lower portion where the rudimentary branchiae are situated. Although the Robber-crab lives ordinarily on land, it must be supposed that these branchiae are of some service; the young are hatched out as ordinary Zoaeas in the sea, and go through a pelagic existence before seeking the land. At the present time the Robber-crab is confined to the Pacific and the islands of the Indian Ocean, wherever the cocoa-nut grows. It seems, however, that its association with the cocoa-nut is a comparatively modern one. Mr. C. Hedley, of Sydney, who has had great experience of the Pacific Islands, informs me that the cocoa-nut is not, as is usually supposed, a native of these coral islands, but has been introduced, probably from Mexico, by the Polynesian mariners before the discovery of America by Columbus. Before the introduction of the cocoa-nut the Robber-crab must have fed on some other tree, possibly the Screw Pine, Pandanus.
The abdomen is full of oil, and is much prized as a delicacy by the natives, who tell many strange legends about the creature, but the philosopher may well find its structure more strange than fiction, and the consideration of its morphology an intellectual feast.
The appearance of the thorax and of the thoracic limbs is thoroughly Pagurid; the structure of the abdomen is highly peculiar.
From the ventral surface (Fig. 119) we can see at the tip of the tail three small calcified plates, which represent the fifth and sixth terga and the telson. Attached to the sixth segment are 175the much reduced and rudimentary pleopods of that segment, and on the left hand side of the body in the female are three well-developed pleopods of the first, second, and third segments, which are used for carrying the eggs. The extraordinary asymmetry of these limbs compared with the complete symmetry of the abdomen itself is only explicable on the hypothesis that these animals are descended from Hermit-crabs which had lost the pleopods on the right side.

Fig. 119.—Birgus latro, ♀, × ⅙, ventral view. Ab, First pleopod; T, last pereiopod.
These appendages are entirely absent in the male. The ventral surface of the abdomen is curiously warty and rugose, and is very soft and pulpy owing to the immense store of oil which it contains.

Fig. 120.—Dorsal view of abdomen, A, of Cenobita, sp.; B, of Birgus latro. T, Telson; 1–6, 1st–6th abdominal segments.
If we look at the dorsal surface of the abdomen we find that, unlike that of the Hermit-crabs, it is completely protected by a number of hard plates (Fig. 120, B). Beneath the carapace can be seen a number of small plates belonging to the last thoracic 176segment; following these there are four large plates (1–4) representing the terga of the first four abdominal segments; the fifth, sixth, and the telson are, as has been stated, carried on the under side of the abdomen, but they are represented diagrammatically (5, 6, T) in the dorsal view. Besides the large terga, there are a number of small plates laterally, usually two to each segment, but they show a tendency to subdivide and increase in the largest specimens. This condition of affairs is very different to that in the naked fleshy abdomen of an ordinary Pagurid, but it can easily be deduced from that of the genus Cenobita, ordinary Hermit-crabs found in the Indo-Pacific Oceans, from which the Robber-crab has evidently descended. In Cenobita (Fig. 120, A) we see the same system of plates upon the dorsal surface of the abdomen, but they are much smaller, and the lateral plates are not so numerous; indeed, the greater part of the abdomen remains fleshy and uncalcified. The under surface of the abdomen shows the same rugosity as is found in Birgus, and from a number of other anatomical characters it is evident that the Robber-crab is a highly modified Cenobita that has deserted its shell and developed a symmetrical abdomen protected by expanded and hardened plates which represent those found in a reduced condition in Cenobita. The species of Cenobita although they inhabit shells and have normal branchiae, live on the shore, and have not been seen to descend actually into the sea.
The Lithodidae, which are found in temperate seas, especially on the Northern Pacific coasts (though Lithodes maia occurs in the North Sea, and certain species inhabit deep water in the Indian Ocean), have a deceptively Brachyuran appearance, the thorax being much shortened and the abdomen being much 177reduced and carried tightly flexed on to the ventral surface of the thorax. They live a free, unprotected existence, and are highly calcified. They are, however, certainly Pagurids, as is evidenced by a number of anatomical characters, but most clearly by the asymmetry of the abdomen, especially in the female, which is not only markedly asymmetrical in the arrangement of its dorsal plates (Fig. 121), but also in the presence of three pleopods upon the left side only, as in Birgus. The male is without these appendages, and the sixth pair of pleopods is absent in both sexes. The remarkable calcified plates upon the abdomen bear a superficial resemblance to those in Birgus, but their evolution is traced, not from a Cenobite, but from an Eupagurine stock.[144]

Fig. 121.—Lithodes maia, ♀, in ventral view, × ¼. The abdomen is flexed on the thorax, so that its dorsal surface is seen. l.3, Lateral plates of third abdominal segment; l.5, left lateral plate of fifth abdominal segment; m, marginal plate; T, brush-like last pereiopod; Te.6, telson and sixth abdominal segment.
In some of the Eupagurinae, e.g. Pylopagurus, feebly calcified plates are present upon the segments of the abdomen (Fig. 122, A).
In the most primitive of the Lithodidae we witness the 178reduction (Fig. 122, B) and disappearance (C) of these original plates, their place being taken first by a number of irregularly situated small spines and warts, which, however, subsequently fuse up to form definite segmental plates. In Lithodes maia, ♂ (D), there are a series of lateral and marginal plates, while in Acantholithus (E) a number of median plates appear, presumably by the fusion of the small spines present in the median line in Lithodes maia; finally, a fusion of the marginal and lateral plates may take place, so that each abdominal segment is covered by a median and two paired lateral plates.

Fig. 122.—Diagrams of abdomen: A, of Pylopagurus, sp.; B, of Hapalogaster cavicauda; C, of Dermaturus hispidus; D, of Lithodes maia, ♂; E, of Acantholithus hystrix. c, Central plates; l, lateral plates; m, marginal plates; T, telson; 1–6, 1st–6th abdominal segments. (After Bouvier.)
It is to be noted that the males and females of the various species do not follow a parallel course of development, the plates in the male being symmetrical, while those of the female are often highly asymmetrical (compare Figs. 122, D, and 121), thus giving the strongest evidence of a Pagurid ancestry.
Birgus and the Lithodidae, then, are Pagurids which have given up living in shells, and have become adapted to a free existence, protecting their soft parts by the development of 179hard plates, and re-acquiring, to a greater or less degree, a secondary symmetry of form. But the story of Pagurid evolution does not apparently stop here. The genus Paralomis, from the West Coast of America, superficially resembles Porcellana, and is held to be descended from such forms as Pylocheles, while isolated species are known (though not well known), such as Tylaspis, described in the Challenger Reports,[145] which appear to be Pagurids that have deserted their shells.

Fig. 123.—Four stages in the development of Eupagurus longicarpus or E. annulipes, × 20. A, Ventral view of Zoaea; B, lateral view of Metazoaea; C, dorsal view of Glaucothoe; D, dorsal view of adolescent stage. Ab.6, 6th abdominal appendage; Mxp.1, Mxp.3, 1st and 3rd maxillipedes. (After M. T. Thompson.)
The metamorphosis of the Hermit-crabs has recently been studied by M. T. Thompson.[146]
The Zoaea (Fig. 123, A) differs from that of the Galatheidea mainly in the absence of the long spines. It possesses the usual 180appendages characteristic of the Zoaea, namely, the first and second antennae, mandibles, first and second maxillae, and two pairs of biramous swimming maxillipedes and small third maxillipedes. In the Metazoaea (B), as in the Anomura generally, the third maxillipedes develop into biramous swimming organs, a thing they never do in the Brachyura, and the rudiments of the thoracic segments put in a first appearance. The abdominal segments are already fully formed in the Zoaea stage, so that here as in all other Zoaeas, the order of development from in front backwards is disturbed by the precocious differentiation of the abdominal segments. The next stage is the “Glaucothoe” (Fig. 123, C), which corresponds to the Megalopa of Brachyura (Fig. 125, p. 183). It differs from the adult Hermit-crab in the perfect symmetry of its body, the segmented abdomen, and the presence of five pairs of normal biramous pleopods. At this stage, which lasts four or five days, it resembles closely a little Galatheid. The asymmetry of the adult (Fig. 123, D) is now imposed upon this larva by the migration of the liver, gonads, and green glands into the abdomen, and by the shifting of the posterior lobes of the liver on to the left side of the intestine, which is displaced dorsally and to the right. The gonad lies entirely on the left side. The pleopods of the right side now degenerate, more completely in the male than in the female, and this degeneration is not completed until the little crab has found a shell and lived in it for some time. If a shell is withheld from it, the degeneration of the pleopods is much retarded, so that although the Hermit-crab assumes its asymmetry without the stimulus of the spiral shell, yet this stimulus is necessary for the normal completion of the later stages.
Fam. 1. Pylochelidae.—The abdomen is macrurous and symmetrical, with all the limbs present. Pylocheles (Fig. 118, p. 173).
Fam. 2. Paguridae.—The abdomen is asymmetrical, with some of the limbs lost. The antennal scale is well developed, and the flagella of the first antennae end in a filament.
Sub-Fam. 1. Eupagurinae.—The third maxillipedes are wide apart at the base, and the right chelipedes are much larger than the left. Parapagurus from deep-sea, Eupagurus from temperate, especially north temperate seas. Pylopagurus.
Sub-Fam. 2. Pagurinae.—The third maxillipedes are approximated 181at the base; the chelipedes are equal or subequal, or the left is much larger. Chiefly in the warm and tropical seas, but Clibanarius and Diogenes also in the Mediterranean.
Fam. 3. Cenobitidae.—The abdomen is as in Paguridae. The antennal scale is reduced, the flagella of the first antennae end bluntly. The members of this family are characteristic of tropical beaches, where they live on the land. Cenobita, with about six species, in the West Indies and Indo-Pacific, living in Mollusc shells; Birgus (Fig. 119) on Indo-Pacific coral islands.
Fam. 4. Lithodidae.—The abdomen is bent under the thorax, and the body is crab-like and calcified. The rostrum is spiniform, and the sixth abdominal appendages are lost.
Sub-Fam. 1. Hapalogasterinae.—Abdomen not fully calcified, and without complicated plates. Hapalogaster and Dermaturus in the North Pacific littoral.
Sub-Fam. 2. Lithodinae.—Abdomen fully calcified, with a complicated arrangement of plates. Lithodes (Fig. 121) practically universal distribution, littoral and deep sea. Acantholithus, deep littoral of Japan; Paralomis, west coast of America. This last genus should probably be placed in a separate family.
Sub-Order 3. Brachyura.[147]
The abdomen is much reduced, especially in the male, and is carried completely flexed on to the ventral face of the thorax so as to be invisible from the dorsal surface. The pleopods in the male are only present on the two anterior segments, and are highly modified as copulatory organs; the pleopods in the female are four in number and are used simply for carrying the eggs; the pleopods of the sixth pair are always absent in both sexes. The first antennae and the stalked eyes can be retracted into special pits excavated in the carapace.

Fig. 124.—A, Zoaea, × 24, and B, Metazoaea, × 13, of Corystes cassivelaunus. Ab, 3rd abdominal segment; An, 1st antenna; E, eye; G, gills; M, 1st maxillipede; T.8, last thoracic appendage. (After Gurney.)

Fig. 125.—Later stage (Megalopa) in the development of Corystes cassivelaunus, × 10. A, Antenna; Ab, 3rd abdominal segment; C, great chela; T.8, last thoracic appendage. (After Gurney.)
The larva hatches out as a Zoaea[148] (Fig. 124, A) very similar to that of the Anomura; it is furnished with an anterior and posterior spine on the carapace. It is characteristic of the Brachyuran Zoaea that the third maxillipede is fashioned from the beginning in its definitive expanded form, and is never a biramous swimming organ as in the Anomura. The only exception to this rule is found in the Dromiacea, the most primitive of the Brachyura, to be soon considered, in which not only the third maxillipede, but also the first pair of pereiopods may be developed as biramous oars, a condition taking one back to the Mysis stage of the Macrura. The Metazoaea (Fig. 124, B) has the rudiments of the thoracic limbs developed and crowded together at the back of the carapace; they are all laid down in their definitive forms, and the abdomen has the pleopods precociously developed. These Zoaeal stages are of course pelagic, but the Metazoaea next passes into the Megalopa stage (Fig. 125), in which the little crab forsakes its pelagic life and assumes the ground-habits of the adult; the Megalopa, which corresponds exactly to 183the Glaucothoe of the Pagurids, resembles a small Galathea or Porcellana, the abdomen being still large and unflexed and furnished with normal pleopods. From this stage the adult structure is soon achieved, though, owing to the continued growth of the Crustacea even after maturity is reached, there is often a slight progressive change in structure, especially in the male, at each successive moult of the individual. The Megalopa of Corystes cassivelaunus is peculiar in the immense production of the second antennae, which act as a respiratory tube (Fig. 125).
The Brachyura must be considered under the following subdivisions:—
Tribe 1. Dromiacea.
All authorities are agreed that these[149] are the most primitive of the Brachyura. In them the abdomen is much less reduced in both sexes than in other Brachyura; there is a common orbitoantennary fossa, into which eyes and antennae are withdrawn, instead of a separate one on each side for each organ; the carapace is often much elongated as in the Macrura and Anomura, and a number of other anatomical characters might be mentioned which characterise the Dromiacea as intermediate between the true Brachyura and the lower forms. There are, however, two views as to the relationship of the Dromiacea; Claus held that they proceeded from a Galatheid 184stock, and hence that the development of the Brachyura ran through an Anomurous strain; but Huxley, and latterly Bouvier,[150] adopt the view that the Dromiacea are descended, not from the Galatheidae, but direct from the Macrura, and especially from the Nephropsidea. Special resemblances are found between the Jurassic Nephropsidae and certain present day Dromiacea, e.g. Homolodromia paradoxa, the detailed form of the carapace in the two cases being very similar. It is, however, a little strange that in the Dromiacea we meet with the same reduction and dorsal position of the last, or last two pairs of thoracic limbs which we saw to be such a characteristic feature of the Anomura, especially of the Galatheidae. In the Dromiacea these limbs may be chelate, and they are used for attaching shells and other bodies temporarily to the back. Must we suppose that this resemblance to the Anomura is due to convergence, or that the Nephropsidae, which gave rise to perhaps both Galatheidae and Dromiacea, had this character, and that it has been subsequently lost in the Macruran stock? We have already mentioned that the Metazoaea of Dromia has not only a well-developed swimming third maxillipede, but also a biramous first pereiopod, a character which speaks strongly for Macruran affinities.

Fig. 126.—Dromia vulgaris, × 1. (After Milne Edwards and Bouvier.)
Fam. 1. Dromiidae.—The eyes and antennules are retractile into orbits. The last two pairs of thoracic limbs are small, and held dorsally. The sixth pair of pleopods are rudimentary or absent. Homolodromia from West Indies, deep-sea. Dromia, widely dispersed. D. vulgaris (Fig. 126) occurs on the English coasts.
Fam. 2. Dynomenidae.—Similar to the preceding family, but only the last pair of thoracic limbs is small, and held dorsally. The sixth pair of pleopods are reduced, but always present. Dynomene in the Indo-Pacific.
Fam. 3. Homolidae.—The eyes and antennules are not retractile into orbits. Only the last pair of thoracic limbs are reduced, the sixth pair of pleopods altogether absent. Homola 185and Latreillia, widely distributed, occur in the Mediterranean. Latreillopsis from the Pacific. L. petterdi,[151] a magnificent species, with the carapace nearly a foot long, and with very long legs like a Spider-crab, has been dredged from 800 fathoms east of Sydney, New South Wales.
Tribe 2. Oxystomata.
This group comprises Crabs whose carapace is more or less circular, while the mouth, instead of being square as in the remaining Brachyura, is triangular with the apex pointing forward, and the third maxillipedes are not expanded into the flattened, lid-like structures found in other Crabs. There is the same tendency in some of the genera for the posterior thoracic limbs to be reduced and carried dorsally, as in the Galatheidae and Dromiacea. The well-known Dorippe from the Mediterranean has this feature, and frequently carries an empty shell upon its back, and Cymonomus[152] presents the same peculiarity.

Fig. 127.—Cymonomus granulatus, × 1. A.1, A.2, 1st and 2nd antennae; E, eye-stalk; S, extra-orbital spine of carapace. (After Lankester.)
Cymonomus granulatus (Fig. 127) is an abyssal form that has been dredged from the Mediterranean and North Atlantic, in which the eye-stalks are curiously tuberculated, and the ommatidia of 186the eye are entirely unpigmented and degenerate, though a few corneal facets are still recognisable. This species is replaced by C. quadratus in the Caribbean Sea and by C. normani on the East African coast, in which the alteration of the eye-stalks into thorny, beak-like projections becomes progressively marked, and all traces even of the corneal facets disappear. This remarkable genus was mentioned in the excursus on Crustacean eyes on p. 149.

Fig. 128.—Calappa granulata, from in front, × ½. C, Hand of chelipede; T, walking legs. (After Garstang.)
The Oxystomata, like the Cyclometopa, to be considered later, live in sandy and gravelly regions, and burrow to a greater or less extent, and we find in both groups admirable adaptations for securing a pure stream of water, uncontaminated by particles of sand, for flushing the gills. Perhaps the most remarkable of these adaptations is afforded by Calappa.[153] This animal has the chelipedes wonderfully modified in structure, and when it is reposing in the sand it holds them apposed to the front of the carapace, as shown in Fig. 128, so that the spines upon their edges, together with the hairy margin of the carapace, form a most efficient filter for straining off sand and grit from the stream of water which is sucked down between the closely-fitting chelipedes and carapace, to enter the branchial chambers at their sides. The exhaled current of water passes out anteriorly through a tube formed by a prolongation of the endopodites of the first maxillipedes. The exhalant aperture is shown in Fig. 128 by the two black cavities below the snout in the middle line.
A similar method is pursued by the related Matuta banksii[153] (Fig. 129), a swimming and fossorial Crab found in the Indo-Pacific. In this Crab the chelipedes also fit against the carapace to form a strainer, and their function is assisted by the enlargement of the posterior spine, which acts as a kind of elbow-rest to keep 187the chelipedes properly in position. The inhalant openings are situated just in front of the chelipedes. It is a most remarkable fact that among the Cyclometopa, Lupa hastata (Fig. 131) has an exactly similar arrangement. Apparently we have here another instance of convergence, similar to that of Corystes and Albunea, but the case is complicated by the fact that some of the Oxystomata, and among them Matuta, show a certain amount of relationship to the Cyclometopous Portunids, so that it is just conceivable that the resemblances in the respiratory arrangement are due to a common descent and not to convergence.

Fig. 129.—Dorsal view of Matuta banksii, × 1. (From an original drawing prepared for Professor Weldon.)
In the Leucosiidae, of which the Mediterranean Ilia nucleus (Fig. 130) is an example, the inhalant aperture is situated between the orbits, and leads into gutters excavated in the “pterygostomial plates” flanking the mouth, which are furnished with filtering hairs and are converted into closed canals by expansions of the exopodites of the third maxillipedes. Thus these Crabs possess a filtering apparatus independent of the chelipedes and of the margin of the carapace.
Fam. 1. Calappidae.—Cephalothorax rounded and crab-like. The abdomen is hidden under the thorax, the antennae are small, and the legs normal in position. The afferent openings to the gill-chambers lie in front of the chelipedes. Male openings on coxae of last pair of legs. Calappa (Fig. 128) circumtropical, 188and extending into the warmer temperate seas. Matuta (Fig. 129) from the Indo-Pacific.

Fig. 130.—Dorsal view of Ilia nucleus, × 1. (From an original drawing prepared for Professor Weldon.)
Fam. 2. Leucosiidae.—Similar to the above, but the afferent openings to the gill-chambers lie at the bases of the third maxillipedes. Male openings on the sternum. This family contains a great number of forms, with headquarters in the tropical littoral, but extending into the temperate seas. Ilia in the European seas. I. nucleus (Fig. 130) common in the Mediterranean. Ebalia in the Atlantic, North Sea, and Indo-Pacific. Leucosia in Indo-Pacific.
Fam. 3. Dorippidae.—Cephalothorax short and square. The abdomen is not hidden under the thorax; the antennae are large, and the last two pairs of legs are held dorsally, and have terminal hooked claws. Dorippe, littoral in Mediterranean and Indo-Pacific. Cymonomus (Fig. 127) from deep-sea of Atlantic and Mediterranean.
Fam. 4. Raninidae.—Similar to Dorippidae, but the cephalothorax is elongated, and the legs usually have the last two joints very broad. Several genera, chiefly in the deeper littoral zone. Ranina dentata in the Indo-Pacific.
Tribe 3. Cyclometopa.
In these Crabs the carapace is circular rather than square; its frontal and lateral margins are produced into spines and there is no pointed rostrum. The mouth is square, and the third maxillipedes are greatly flattened and form a lid-like expansion over the other oral appendages. This group includes the common Shore-crab of our coasts (Carcinus maenas), the swimming Crabs with expanded pereiopods (Portunus, Lupa, etc.), the Edible Crab (Cancer pagurus), and many others.
Corystes cassivelaunus is a Crab of doubtful affinities. It is sometimes placed among the Oxyrhyncha, but, as Gurney[154] has 189pointed out, the Megalopa shows Portunid characters, and the resemblance to the Oxystomata in the front of the carapace and in the mouth may be secondary. The respiratory arrangement of this Crab has already been mentioned in comparing its structure with that of the Mole-crab Albunea. The form of the antennal tube can be gathered from the figure of the Megalopa stage (Fig. 125, p. 183). It should be noted that when the Crab is buried in the sand with only the tip of the antennal tube projecting, the water is sucked down and enters the branchial cavities anteriorly, the antennal tube being continued by a tube formed from the third maxillipedes and the forehead; the water is exhaled at the sides of the branchial cavities beneath the branchiostegites. Thus in Corystes the normal direction of the current is reversed, but when the Crab is not buried, and is moving over the surface, it breathes in the usual manner, taking in the water at the sides of the branchiostegites and exhaling it anteriorly by the tube. The related Atelecyclus, found like Corystes very commonly at Plymouth, uses two methods of breathing: when it is in the surface-layers of sand it makes use of its antennal tube, which is, however, much shorter than in Corystes; but when it burrows deeper, where the antennal tube is no use, it folds its chelipedes and also its other legs, which are densely covered with bristles, so as to form a reservoir of pure water underneath it free from sand, which it passes through the gill-chambers in the usual manner (see Garstang, loc. cit. p. 186).
The respiratory adaptations in Lupa hastata and their convergence towards those of the Oxystomatous Matuta have been already touched upon (pp. 186, 187).
In this connexion must be mentioned the interesting experiments of W. F. R. Weldon[155] upon the respiratory functions of Carcinus maenas at Plymouth, since these were the first noteworthy observations directed towards the exact measurement of the action of natural selection upon any animal, a field of observation in which Weldon will always be looked upon as a pioneer. An extended series of measurements by Weldon and Thompson on male specimens of Carcinus maenas of various sizes between the years 1893 and 1898 showed a steady decrease in the ratio of carapace breadth to length; the Crabs appeared to be becoming steadily narrower across the frontal margin, and 190the same thing, though not to the same extent, was happening in female Crabs. Weldon supposed that this change might be correlated with the silting up of Plymouth Sound and the consequent fouling of the water. To test this hypothesis he kept a very large number of male Crabs in water to which fine porcelain clay was added and kept in continual motion. In the course of the experiments the survivors and the dead were measured, and it was found that the mean carapace breadth of the survivors was less than that of those that succumbed. The experiment was repeated with the fine sand that is deposited and left at low water upon the stones on Plymouth beach, and the same result was observed. It was also noticed that the individuals which died had their gills clogged with the sand, while those that survived had not. As a further confirmation, a great many young male Crabs were isolated and kept in pure filtered water, and they were measured before and after moulting; these measurements, when compared with measurements of the frontal breadth in Crabs of the same size taken at random upon the beach, were found to show a greater breadth than the wild Crabs, thus indicating that a selection of narrow Crabs was taking place in Nature which did not take place when the Crabs were protected from the effects of fine sand in the water.
The whole chain of evidence goes to show that the carapace breadth in Carcinus maenas in Plymouth Sound is being influenced by the rapid change of conditions occurring in the locality. Various objections have been urged against this conclusion, but, though they merit further investigation, they do not appear very weighty.
The fresh-water Crab, Thelphusa fluviatilis, common in the South of Europe and on the North coast of Africa, belongs to the Cyclometopa, and is interesting from its direct mode of development without metamorphosis.
Fam. 1. Corystidae.—The orbits are formed, but, unlike all the other families of the Cyclometopa, are incomplete. The body is elongate and oval, and the rostrum and front edge of the mouth rather as in the Oxyrhyncha, in which Tribe they are sometimes included. Corystes, with a few species in European seas. C. cassivelaunus at Plymouth.
Fam. 2. Atelecyclidae.—Perhaps related to the foregoing. 191The carapace is sub-circular, and the rostrum short and toothed. Atelecyclus, European seas.
Fam. 3. Cancridae.—The carapace is broadly oval or hexagonal, and the flagella of the second antennae are short and not hairy as in the foregoing. The first antennae fold lengthwise. Carcinus maenas on English and North European coasts. This crab has become naturalised in some unexplained manner in Port Phillip, Melbourne. Cancer in North Atlantic, North Pacific, and along the west coast of America into the Antarctic regions. C. pagurus is the British Edible Crab.

Fig. 131.—Dorsal view of Lupa hastata, × 1. (From an original drawing prepared for Professor Weldon.)
Fam. 4. Portunidae.—The legs are flattened and adapted for swimming. The first antennae fold back transversely. Portunus, Atlantic and Mediterranean. Neptunus, Indo-Pacific. Callinectes, C. sapidus, the edible blue Crab of the Atlantic coasts of America. Lupa (Fig. 131).
Fam. 5. Xanthidae.—The first antennae fold transversely, but the legs are not adapted for swimming; the body is usually transversely oval. This family is especially characteristic of the tropical littoral, where it is very widely represented. Xantho, Actaea, Chlorodius, Pilumnus, Eriphia, with E. spinifrons, common in the Mediterranean.
Fam. 6. Thelphusidae (Potamonidae).—Fresh-water crabs, with the branchial region very much swollen. Thelphusa (or Potamon) has nearly a hundred species distributed from North Australia, through Asia, Japan, the Mediterranean region, and throughout Africa. Potamocarcinus in tropical America.
Tribe 4. Oxyrhyncha.
This section includes the Spider-crabs and related genera, in which the carapace is triangular, with the apex in front 192formed by a sharply-pointed rostrum. There are two chief series, the one comprising the Spider-crabs, with much elongated walking legs, e.g. the huge Maia squinado of European seas, the yet more enormous Macrocheira kämpferi from Japan, supposed to be the largest Crustacean in existence, and sometimes spanning from outstretched chela to chela as much as eleven feet, and the smaller forms, such as Inachus, Hyas, and Stenorhynchus, which are so common in moderate depths off the English coasts. The other series is represented by genera like Lambrus (Fig. 133), in which the legs are not much elongated, but the chelipedes are enormous.
The Spider-crabs do not burrow, and their respiratory mechanism is simple; but since they are forms that clamber about among weeds, etc., upon the sea-bottom, they often show remarkable protective resemblances to their surroundings, which are not found in the burrowing Cyclometopa. Alcock[156] gives a good account and figure of Parthenope investigatoris, one of the short-legged Oxyrhyncha, the whole of whose dorsal surface is wonderfully sculptured to resemble a piece of the old corroded coral among which it lives.
But besides this, the long-legged forms, such as Inachus, Hyas, etc., have the habit of planting out Zoophytes, Sponges, and Algae upon their spiny carapaces, so that they literally become part and parcel of the organic surroundings among which they live. It may, perhaps, be wondered what are the enemies which these armoured Crustacea fear. Predaceous fish, such as the Cod, devour large quantities of Crabs, which are often found in their stomachs; and Octopuses of all sorts live specially upon Crabs, which they first of all paralyse by injecting them with the secretion of poison-glands situated in their mouth. The poison has been recently found by Dr. Martin Henze at Naples to be an alkaloid, minute quantities of which, when injected into a Crab, completely paralyse it. When the Crab is rendered helpless the Octopus cuts out a hole in the carapace with its beak, and sucks all the internal organs, and then leaves the empty shell.
Many of the Oxyrhyncha are found in the abysses; among them are Encephaloides armstrongi (Fig. 132), dredged by Alcock from below the 100–fathom line in the Indian Ocean, which has 193the gill-chambers (G) greatly swollen and enlarged to make up for the scarcity of oxygen in these deep regions.

Fig. 132.—Encephaloides armstrongi, × 1. The long walking legs are omitted. C, Great chela; G, one of the greatly swollen gill-chambers. (After Alcock.)
Fam. 1. Maiidae.—The chelipedes are not much larger than the other legs, but are very mobile. Orbits incomplete. A very large family, including all the true Spider-crabs, very common in the Atlantic and Mediterranean littoral. Inachus, Pisa, Hyas, Stenorhynchus, Maia, Encephaloides (Fig. 132).
Fam. 2. Parthenopidae.—The chelipedes are much larger than the other legs. Orbits complete. Lambrus (Fig. 133), Parthenope.

Fig. 133.—Lambrus miersi, × 1. (After Milne Edwards and Bouvier.)
Fam. 3. Hymenosomatidae. The carapace is thin and flat; the chelipedes are neither very long nor especially mobile. There are no orbits, and the male openings are on the sternum. Characteristic of the Antarctic seas. Hymenosoma, Trigonoplax.
Tribe 5. Catometopa.
These Crabs resemble the Cyclometopa in general appearance, but the carapace is very square in outline, and its margins are never so well provided with spines as in the Cyclometopa. The position of the male genital openings is peculiar, since they lie upon the sternum, and are connected with the copulatory appendages upon the abdomen by means of furrows excavated in the sternum. The Catometopa are either littoral or shallow water forms, or else they live entirely on land. The Grapsidae are marine Crabs, Pachygrapsus marmoratus (Fig. 134) at Naples being exceedingly common on rocks at high-water mark, over which it scuttles at a great rate; in the Mediterranean it takes 194the place of our common Garcinus maenas, which is not found there.

Fig. 134.—Dorsal view of Pachygrapsus marmoratus, × ⅓. (From an original drawing prepared for Professor Weldon.)
Among the land genera are Ocypoda, Gelasimus, and Gecarcinus of tropical lagoons and coastal swamps. Ocypoda often occurs in vast crowds in these regions, and digs burrows in the sand.

Fig. 135.—Gelasimus annulipes, × 1. A, Female; B, male. (After Alcock.)
Gelasimus (Fig. 135) is remarkable for the enormous size of one of the chelipedes, generally the right, in the male, which may actually exceed in size the rest of the body. It is not known what purpose this organ serves in the various species. In Gelasimus it is supposed that the male stops up the mouth of the burrow with it when he and the female are safely inside. It is also used as a weapon in sexual combats with other males; but Alcock, from observations made in the Indian Ocean, believes that the males use it for exciting the admiration of the females in courtship, as the huge chela is bright red in colour, and the males brandish it about before the females as if displaying its florid beauty.
The species of Ocypoda are exclusively terrestrial, and cannot live for a day in water. The gills have entirely disappeared, and the branchial chambers are converted into air-breathing lungs with highly vascular walls, the entrances into which are situated as round holes between the bases of the third and fourth pairs of walking legs. As their name implies, they can run with 195astonishing rapidity, and they seem to be always on the alert, directing their eyes, which are placed on exceedingly long stalks, in all directions.
Some of the Grapsidae, e.g. Aratus pisonii, are partially adapted for life on land. Fritz Müller, in his Facts for Darwin, alludes to this creature as “a charming lively crab which ascends mangrove bushes and gnaws their leaves.” The carapace can be elevated and depressed posteriorly, apparently by means of a membranous sac, which can be inflated by the body-fluids. This Crab retains its gills and can breathe under water in the ordinary way.
A great many other Catometopa are land-crabs; but we may specially mention the genus Gecarcinus, related to the marine Grapsidae, which has representatives in the West Indies and West Africa. The Crabs of this genus may live in sheltered situations several miles from the sea, but in spring the whole adult population rushes down in immense troops to the shore, where breeding and spawning take place; and when this is completed they migrate back again to the land. The young pass through the normal larval stages in the sea and then migrate inland.[157]
Fam. 1. Carcinoplacidae.—The carapace is rounded and broader than long, usually with toothed front margin. The orbits and eyes are normal, and not much enlarged. Geryon, in the deep littoral of the northern hemisphere. Euryplax, Panoplax, etc., in the American coastal waters. Typhlocarcinus, etc., in the Indo-Pacific.
Fam. 2. Gonoplacidae.—The carapace is square, with the antero-lateral corners produced into spines. The orbits are transversely widened, and the eye-stalks long. Gonoplax, widely distributed in the littoral zone. G. rhomboides in British and European seas.
Fam. 3. Pinnotheridae.—Carapace round, with indistinct frontal margin. Orbits and eyes very small, often rudimentary. The members of this family live symbiotically or parasitically in the shells of living Bivalve Molluscs, corals, and wormtubes in all seas except the Arctic. Pinnotheres pisum is fairly 196commonly met with off the English coasts in the mantle-cavity of Cardium norwegicum.
Fam. 4. Grapsidae.[158]—Carapace square, the lateral margins either strictly parallel or slightly arched. The orbits and eyes are moderately large, but the eye-stalks are not much lengthened. Littoral, fresh-water, and land. Pachygrapsus marmoratus (Fig. 134), the common shore-crab of the Mediterranean. Sesarma, with fresh-water and land representatives in the tropics of both hemispheres. Cyclograpsus, marine in the tropical littoral.
Fam. 5. Gecarcinidae.—Carapace square, but much swollen in the branchial region. Orbits and eyes moderately large. Typically land forms, which only occasionally visit the sea or fresh water. Cardisoma is a completely circumtropical genus, with species in tropical America, West and East Africa, and throughout the Indo-Pacific. Gecarcinus in West Indies and West Africa.
Fam. 6. Ocypodidae.—Carapace square or rounded, generally without teeth on the lateral margins. The orbits transversely lengthened, eye-stalks usually very long. The members of this family generally inhabit the mud-flats and sands of tropical coasts; in the southern hemisphere they extend far into the temperate regions. Macrophthalmus, with numerous species, in Indo-Pacific. Gelasimus (Fig. 135), in the tropics of both hemispheres. Ocypoda, with similar distribution.
CHAPTER VII
REMARKS ON THE DISTRIBUTION OF MARINE AND FRESH-WATER CRUSTACEA
A. Marine.
The great majority of the Crustacea are inhabitants of the sea. From a Zoogeographical point of view we divide the sea into three chief regions, each of which is characterised by a special kind of fauna—the littoral, the pelagic, and the abyssal regions.
The littoral region, which comprises all the shallow coastal waters down to about 100 fathoms, varies very greatly in its physical character according to the nature of the coast, its geological constitution, latitude, etc., but, on the whole, it is characterised by variability of temperature and salinity, by the presence of sunlight, and by the continuous motion of its waves. On the shores of the large oceans this region is also greatly affected by the tides. It is inhabited by a vast assemblage of Crustacea, all of which are dependent upon a solid substratum, either of rock or sand, or of vegetable or animal growth, upon which they may wander in search of food, or in which they may hide themselves. In consequence, the character of the Crustacea on any shore is largely determined by its geological nature.
Although a certain number of Entomostraca such as Copepoda (Harpacticidae and Cyclopidae), Ostracoda (Cypridae and Cytheridae), and a few Operculata are littoral in habit, it is the Malacostraca, from their larger size and variety of form, which give the character to coastal waters.
On rocky coasts, especially those affected by tides, a great many kinds of Shore-crab are found, which hide at low tide in 198the rock-pools and under stones. Carcinus maenas is characteristic of the rocky coasts of the North Sea, while it is replaced in warmer seas and all round the tropics by Crabs of the family Grapsidae, which are typical rock-livers, and exceedingly agile in clambering over tide-washed rocks. Porcellanidae are also very common under stones at low tide on rocky beaches. Such typical Shore-crabs as these are remarkably resistant to desiccation, and can live out of water for an astonishing time; nor do they require a change of water provided they have access to the air. The edible crab (Cancer pagurus) and the lobsters (Homarus and Palinurus) are dependent on rocks, but they rarely come close in-shore, preferring depths of a few fathoms.
Sandy coasts are preferred by Shrimps and Prawns, which haunt the shallow coastal waters in shoals; and in the sand are found all the Crabs whose respiratory mechanism is specially adapted for life in these regions, e.g. Hippidea or Mole-crabs, Corystes, Matuta, Calappa, etc.
Characteristic of sandy bottoms are also the Thalassinidea, such as Callianassa, which excavate galleries in the sand. On tropical sandy shores various species of Ocypoda and Gelasimus are conspicuous, which have deserted the sea, and live in burrows which they excavate on the shore. Gelasimus is especially abundant in the muddy sand of tropical mangrove swamps.
Besides the rocky and sandy coasts we must distinguish the muddy shores and bottoms which support a large amount of vegetable and animal growth. These, besides harbouring the greater number of Amphipods and Isopods, are also the natural home of the Dromiacea and Oxyrhyncha, or Spider-crabs, among which the habit is common of decking themselves out with pieces of weed or animal growth in order to harmonise better with their surroundings. Pagurids are also especially abundant in the deeper waters of these coasts.
Coral-reefs support a characteristic Crustacean fauna. In the growing coral at the reef-edge a number of small Cyclometopa are found, e.g. Chlorodius, Actaea, Xantho, which are finely sculptured and often coloured so as to harmonise with the coral. Alpheidae also, Shrimp-like Macrura with highly asymmetrical claws, which can emit a sharp cracking sound with the larger claw, are commonly found in pools on the reef. In the coralshingle 199formed by abrasion from the reef-edge at a few fathoms depth, Leucosiidae are found, in which, again, respiratory mechanisms for filtering sand from the gills are present.
Besides the geological nature of the coast, latitude has a very important bearing upon the distribution of littoral Crustacea. Indeed, the present distribution of littoral Crustacea appears to be far more determined by the temperature of the coastal waters than by the presence of any land-barriers, however formidable. We may distinguish an Arctic, Antarctic, and Circumtropical zone.
The Arctic zone includes the true Arctic seas, and stretches right down through boreal regions towards the sub-tropical seas. Almost all the truly Arctic forms penetrate fairly far south, the Arctic seas being characterised more by the absence of temperate forms than by the presence of forms peculiar to itself. At the same time it must be noted that the individuals from the coldest regions often grow to an enormous size, a characteristic which is physiologically unexplained.
A great many of the Crustacea characteristic of this region are circumpolar, i.e. they are not restricted in range to either the Atlantic or Pacific. This is especially true of the extremely northern types, e.g. Crangonidae and Hippolytidae, but it is also true of a number of Crustacea which do not now occur as far north as Greenland or Bering Strait, so that there is no longer any free communication for them between Pacific and Atlantic. This gives rise to a discontinuous distribution in the two oceans, exemplified in the common Shrimp, Crangon vulgaris, which is found on the temperate European coasts and on the Pacific coasts of Japan and Eastern America. The same is true of Eupagurus pubescens and E. bernhardus.
At the same time the boreal Atlantic and Pacific have their peculiar forms. Thus the European and American Lobsters are confined to the Atlantic, while the North Pacific possesses a very rich array of Lithodinae, which cannot be paralleled in the Atlantic.
We may explain the community of many littoral forms to both the North Atlantic and Pacific coasts by the continuous coast-line uniting them, which in former times possibly did not lie so far north, or else was not subjected to so rigorous a climate as now.
200In the Antarctic zone we are presented with very different relations, since the great continents are drawn out to points towards the south, and are isolated by vast tracts of intervening deep sea. Nevertheless, certain littoral forms are circumpolar, e.g. the Palinurid Iasus and the Crabs Cyclograpsus and Hymenosoma. The genus Dromidia is common to Australia and South Africa, though it is apparently absent from South America.
The Isopod genus Serolis is confined to Antarctic seas. The majority are littoral species, and they are distributed round the coasts of Patagonia, Australia, and Kerguelen in a manner that certainly suggests a closer connection between these shores in the past. These facts are, on the whole, evidence in favour of the former existence of an Antarctic continent stretching farther north and connecting Australia, Africa, and S. America—a supposition that has been put forward to account for the distribution of the Penguins, Struthious birds, Oligochaets, Crayfishes, etc., in these regions (see pp. 215–217).
In considering the Arctic and Antarctic faunas the supposed phenomenon of bipolarity must be mentioned, i.e. the occurrence of particular species in Arctic and Antarctic seas, but not in the intermediate regions. This discontinuous type of distribution was upheld for a variety of marine animals by Pfeffer, Murray, and others, but it has been very adversely criticised by Ortmann.[159] As far as the Arctic and Antarctic Decapod fauna in general are concerned, the north polar forms are quite distinct from the south polar. Typical of the former are Hippolyte, Sclerocrangon, Hyas, Homarus, etc.; of the latter, Hymenosoma, Dromidia, Iasus. It appears, however, that in certain special cases, bipolarity of distribution may be produced owing to the operation of peculiar causes. Two such cases seem to be fairly well established. Crangon antarcticus occurs at the two poles, and apparently not in the intermediate regions; but, as Ortmann points out, it is represented right down the West American coast by a very closely related form, C. franciscorum. The waters on the tropical western coasts both of Africa and America are exceedingly cool, and it appears that in this way the Crangon may have migrated across the tropical belt, leaving a slightly modified race to represent it in this intermediate region. The other case of bipolarity is afforded by the “Schizopod,” Boreomysis 201scyphops, which occurs at both poles, but is not known from the tropics. This is a pelagic species, and we know that the Mysidae often descend to considerable depths. We also know that the Mysidae are dependent on cold water, only occurring in boreal or temperate waters. We may safely suppose, therefore, that the migration of this species has taken place by their forsaking the surface-waters as the tropics were approached, and passing down into the depths where the temperature is constantly low even in the tropics.
The dependence of Crustacea upon the temperature of the water is also illustrated by the distribution of the Lithodinae. The headquarters of this family are in the boreal Pacific, with a few scattered representatives in the boreal Atlantic. The cool currents on the western coasts of America, however, have permitted certain forms to migrate as far south as Patagonia, where they still have a littoral habit. In the tropical Indo-Pacific, where a few species occur, they are only found in deep waters. Thus at these various latitudes, by following cool currents or migrating into deep water, they are always subjected to similar conditions of temperature. The same kind of thing is observed in Arctic seas, where deep-sea forms are apt to take on secondarily a littoral habit owing to the temperature of the depths and of the shore being the same.
Despite the impassable barriers of land which now sever the tropical oceans, we can yet speak of a circumtropical zone possessing many species common to its most widely separated parts. Such circumtropical species, occurring on both the Atlantic and Pacific coasts of tropical America, on the West African coast, and in the Indo-Pacific, are various Grapsidae, Calappa granulata and its allies, and certain Albunea. The most striking instance of all is that of the Land-crabs. Of Ocypoda, the greater number of species occur in the Indo-Pacific, but representatives are also found on the tropical Eastern and Western American coasts and on the West African coast, and the same is true of Gelasimus. The genus Cardisoma, belonging to a different group of Land-crabs, is also typically circumtropical.
For this community of the circumtropical species we may certainly advance in explanation the comparatively recent formation of the Isthmus of Panama. Besides the resemblance of the Crustacea on the east and west coasts of the isthmus, we have an 202actual identity of species in several cases, e.g. Pachycheles panamensis and Hippa emerita, and the same thing has been observed for the marine fish.
Another connexion, at any rate during early tertiary times, which probably existed between now isolated tropical coasts, was across the Atlantic from the West Indies to the Mediterranean and West African coasts. Numerous facts speak for this connexion. Species of Palinurus and Dromia occur in the West Indies and the Mediterranean, which only differ from one another in detail, and a connexion between these two regions has been urged from the minute resemblances of the late Cretaceous Corals of the West Indies with those of the Gosau beds of S. Europe, and also of the Miocene land-molluscs of S. Europe with those at the present time found in the West Indies.
To account, then, for the present distribution of littoral Crustacea we must imagine that great changes have taken place during comparatively recent times in the coast-lines of the ocean, but the guiding principle in both the past and present has been temperature, and this factor enables us, despite the immense changes in the configuration of the globe that must have taken place, to divide the coasts latitudinally into Arctic, Antarctic, and Circumtropical zones.
Pelagic Crustacea belong chiefly to the Copepoda (Calanidae, Centropagidae, Candacidae, Pontellidae, Corycaeidae), a few Ostracoda (Halocypridae and Cypridinae), and among Malacostraca a few Amphipoda (Hyperina), some “Schizopoda,” and among Decapoda only the Sergestidae, if we except the few special forms which live on the floating weeds of the Sargasso Sea, e.g. the Prawns Virbius acuminatus and Latreutes ensiferus, and the Brachyura Neptunus sayi and Planes minutus. Besides these Crustacea which are pelagic as adults, there is an enormous host of larval forms, both among Entomostraca and Malacostraca, which are taken in the surface-plankton.
In dealing with the Copepoda we have already mentioned the vast pelagic shoals of these organisms which occur at particular times of the year, and have an important influence on fishing industries. Anomalocera pattersoni (Fig. 27, p. 60) is a good instance of this. It is a large Heterarthrandrian, about 3 mm. long, with the body of a fine bluish green colour; it has a remarkable power of springing out of the water, so that a shoal has the 203appearance of fine rain upon the surface of the sea. It occurs in the open Atlantic and Mediterranean, but comes into the coasts during violent storms; the Norwegian fishermen hail its presence in the fjords as the sign of the approach of the summer herring.
It was Haeckel[160] who first clearly distinguished between “neritic” plankton, the species of which have their centres of distribution in shallow coastal waters and die out gradually as the open ocean is approached, and “oceanic” plankton which is habitually found in the open sea, and though it may invade the coasts is not dependent on the sea-bottom in any way. It appears that although these two kinds of plankton may get mixed up by currents and storms, they are always recruited by new generations from the neritic or oceanic stations proper to each kind.
Common oceanic species, found chiefly in the open Atlantic and in the North Sea, are Anomalocera pattersoni, Calanus finmarchicus, Centropages typicus, Metridia lucens, Oithona plumifera, etc. Common neritic species in the Channel and other coastal waters are Centropages hamatus, Euterpe acutifrons, Oithona nana, Temora longicornis, etc. It was found by Gough[161] that although the true oceanic species invade the Channel from the open Atlantic to the west, they become rarer and rarer as they advance up the Channel. Thus the plankton midway between the Lizard and Ushant at all times of year is about 70 per cent. oceanic, while at the line drawn from Portland to the Cap de la Hague it is about 35 per cent. Seasonal changes in the salinity of the Channel water, chiefly due to the influx of oceanic water from the Atlantic, as observed by Matthews,[162] do not clearly influence the distribution of oceanic and neritic forms. The influx of highly saline water from the Atlantic was most marked during the winter months up to February. From February to May the highly saline water receded, and during the summer months at the line drawn between Portland and the Cap de la Hague the salinity was rather low. This was increased in November by a patch of oceanic water being cut off from the main mass and passing up Channel, and it is noteworthy that during this month 204the highest percentage of oceanic forms was taken in the plankton of this region.
Calanus finmarchicus affords a clear instance of the way in which the plankton may be carried about for great distances by means of currents. This species has its home in the subarctic seas, but is carried down in the spring by the East Icelandic Polar stream to its spawning-place south of Iceland; the enormous shoals produced here are carried back, continually multiplying, along the coasts of Norway during the summer and autumn.
Besides these great migrations, the plankton organisms perform daily movements, the majority of the Crustacea avoiding the surface during the day, and often going down to as much as seventy fathoms or more, and only coming up to the surface at night. Others, however, e.g. Calanus finmarchicus, behave in the converse manner, preferring the sunlit surface to swim in.
Owing to their dispersal by means of oceanic currents the pelagic Crustacea do not offer any very striking features in regard to their distribution, and the possibility of always finding congenial temperatures by passing into the upper or under strata of water enables them to live in almost all seas. The tropical species of Sergestidae are mostly circumtropical, i.e. unhindered by the present barriers of land.
The Abyssal regions of the sea contain many of the most interesting Crustacea. Families entirely confined to the abyss are the Eryonidae, Pylochelidae, and certain Caridean Prawns (Psalidopodidae, etc.), but there are a great number of normally littoral genera which have representatives in deep water. If we draw the limit between the littoral and abyssal regions at about 200 metres, we can characterise the latter as absolutely dark except for the presence of phosphorescent organisms, with the temperature at a little above zero, and with a comparative lack of dissolved oxygen in the water. These conditions bring about remarkable modifications in the structure and life-histories of the inhabitants of the deep sea; we have already touched on the modifications of the visual organs and on the presence of phosphorescence in many of the animals; other points to be noticed are the usually uniform yellowish or bright red coloration, the frequent delicacy of the tissues without much calcification, variations in the structure of the breathing organs, e.g. in 205Bathynomus giganteus and Encephaloides armstrongi, and the loss of the larval development. Owing to the similarity of conditions in the deep sea all over the globe most of its inhabitants are universally distributed. It is also a striking fact that species are found in the deep sea of the tropics whose nearest allies occur, not in the littoral seas of the tropics, but in those of the temperate region. This fact has already been alluded to in dealing with the distribution of the Lithodinae. Alcock[163] remarks that between 50–500 fathoms in the Indian Ocean are found Crabs such as Maia, Latreillia, and Homola, regarded as characteristic of the north temperate seas; the lobster Nephrops andamanica, taken at 150–400 fathoms, is closely allied to the Norwegian N. norwegica; and nine species of “Schizopoda,” which are certainly temperate forms, occur in the Indian Ocean at depths of 500–1750 fathoms.
B. Fresh-Water.[164]
If we except the Crayfishes and River-crabs, the Crustacean fauna of running water is exceedingly poor, but in all standing fresh-water, from the smallest pond to the large lakes and inland seas, Crustacea, especially Entomostraca, are abundant and characteristic, and form an important item in the food of fresh-water fishes. In small ponds a vast assemblage of Cladocera is met with; these animals multiply with great rapidity by parthenogenesis, especially during spring and summer, but on the advent of untoward conditions sexual individuals are produced, which lay fertilised winter-eggs which lie dormant until favourable conditions again arise. As Weismann first pointed out, the frequency with which sexual individuals are produced in the various species is closely correlated with the liability of the water in which they live to dry up; so that the Cladocera which inhabit small ponds usually have at least two “epidemics” of sexual individuals, one during early summer and the other before the onset of winter.
Besides Cladocera, the Phyllopoda (e.g. Apus, Artemia, etc.) 206inhabit small pools; and also a great number of Cyclopidae. Of the other fresh-water families of Copepoda, viz. Centropagidae and Harpacticidae, inhabitants of small pieces of water are Diaptomus castor, as opposed to the other species of Diaptomus which are pelagic, and a number of Harpacticidae (Canthocamptus), the members of this family living in the weed or mud of either small ponds or else on the shores of the larger lakes. The greater number of Ostracoda are found in similar situations.
A district like the Broads of Norfolk, which consists partly of slowly-moving streams and partly of extensive stretches of shallow water, supports a Crustacean fauna intermediate in character between that found in small ponds and the truly pelagic fauna characteristic of deep lakes. A very complete list of the Crustacea of the Norfolk Broads, with an interesting commentary on their distribution, is given by Mr. Robert Gurney.[165] We miss here the pelagic Cladocera, such as Leptodora, Bythotrephes, Holopedium, etc., which form so characteristic a feature of large lakes; at the same time, besides a rich development of the Cladocera, Cyclopidae, and Harpacticidae, which haunt the weeds and mud of shallow waters, we find such species as Polyphemus pediculus and Bosmina longirostris among Cladocera, which are otherwise confined to large bodies of water, and a few pelagic Diaptomus, e.g. D. gracilis. The fauna is also complicated in this district by the proximity to the sea and the frequently high salinity of the water, which allows a number of typically marine Copepods to pass up the estuaries and intermingle with typically fresh-water species; such are Eurytemora affinis among the Centropagidae, and several species of Harpacticidae (see p. 62).
The large lakes of the world, such as the continental lakes of Europe and America, or of our own Lake District, reproduce on a small scale the varied conditions which appertain to the ocean—as in the ocean, we can recognise in these lakes a littoral, a pelagic, and an abyssal region. Our knowledge of the physiography of lakes is largely due to the classical work of Forel,[166] and the following account of the physical conditions in the various regions is condensed from his book.
The littoral region is sharply marked off from the others by the relative instability of its physical conditions, owing to the 207agitation of its waters, the affluence of streams and drainage, and the constant changes of temperature. The water in this region generally contains a good deal of solid matter in suspension, while the shelving banks of the lake support a wealth of vegetable growth, both of Algae and of Phanerogams, down to about 20–25 metres. At this depth the daylight does not penetrate sufficiently to admit of the growth of green plants, so that this region marks the limit, both physical and biological, between the littoral and the abyssal zones. In this littoral region there flourish a great quantity of Entomostraca, most of which are also found in small ponds where similar conditions of life prevail—the pelagic species only penetrating rarely, and by accident, into its waters. At the beginning of July Mr. H. O. S. Gibson and myself found that the weedy littoral region of Grasmere contained almost entirely large quantities of the Cladoceran Eurycercus lamellatus, and a number of Cyclops fuscus and C. strenuus. In the littoral zone of large lakes, Amphipods, Isopods, and fresh-water shrimps may also be met with, but this applies more to the lakes of the Tropics and of the Southern Hemisphere.
The pelagic[167] region is distinguished from the littoral by the greater purity and transparency of its waters, and by the relative stability of the temperature, the annual range, even at the surface, in Geneva being from 4°–20° C., while at 100 metres the water has a uniform temperature of 4° or 5° C. The upper strata are, of course, brightly illuminated, but at 20 metres there is hardly sufficient light for green plants to grow, and at 100 metres it is completely dark. The inhabitants of this region, known collectively as plankton, spend their whole life swimming freely in the water, sometimes at the surface and sometimes in the deeper strata. They consist chiefly of Diatoms, Protozoa, Rotifera, and Crustacea. The pelagic Crustacea, especially the Cladocera, are often the most curiously and delicately built creatures. Leptodora hyalina, which is quite transparent, is the largest of them, attaining to three-quarters of an inch in length, though Bythotrephes longimanus is nearly as large if we include the immense spine which terminates the body. Holopedium gibberum, which is 208the commonest of all in Grasmere lake, but not so frequently met with in the other English lakes, is peculiar in that its body is enveloped in a spherical mass of transparent jelly, sometimes a quarter of an inch in diameter, so that the contents of a tow-net jar full of Holopedium have something of the consistency of boiled sago. The enormous quantities in which these animals often occur during summer is very astonishing; but to be truly appreciated tow-nettings should be taken at the surface of the lake either during night-time when there is not much moonlight, or else on a dark still day when there is a quiet drizzle falling on the surface of the water. In bright sunshine the plankton passes below the surface into the lower strata, and can be usually taken by sinking the tow-net some 10–20 feet, or to even greater depths in the water. The exact reason of these periodic migrations out of the light, and their dependence on other physical conditions, such as temperature and the agitation of the water, is not clearly understood. It appears, however, that when the water is rough, plankton always passes into the deeper regions. Besides the species mentioned, the minute Bosminidae, whose trunked heads are suggestive under the microscope of elephants, and Polyphemus pediculus are among the commonest pelagic Cladocera, though neither Polyphemus nor Bythotrephes ever form shoals. The above-mentioned genera are characteristic of the larger lakes in the Northern Hemisphere. Our knowledge of the Crustacean plankton of tropical lakes and of those of the Southern Hemisphere is limited (but see p. 216).
A very important constituent of lake-plankton is furnished by the Copepoda, especially of the genus Diaptomus. With the exception of Holopedium, by far the commonest Crustacean in Grasmere during July was found by Mr. Gibson and myself to be D. caeruleus.
At the same time a number of Cyclopidae, e.g. Cyclops strenuus, may occur in the pelagic region in considerable quantities, though they were never found by us in such numbers as Diaptomus.
The life-cycle of the pelagic Entomostraca has been studied in both the Cladocera and the Copepoda. In some of the Cladocera Weismann at first supposed that males had altogether disappeared, and that reproduction was entirely parthenogenetic. It appears, however, that all the pelagic species have at least one sexual period, namely, in the autumn, when resting eggs are 209produced which lie dormant during the winter. The pelagic Copepods may either produce resting eggs for the winter (Diaptomus), or else the winter is passed through in the Nauplius stage, the larvae hibernating in the mud until the spring (Cyclopidae).
We have so far only dealt with fresh-water Entomostraca. There are, in addition, a number of Malacostraca which inhabit fresh water, and some of these are found in the abyssal region of the great lakes, which must now be considered.
The physical conditions of the abyssal region are still more stable than those of the pelagic region, since the water is never disturbed, the bottom is always composed of a fine mud, the temperature is constant at 4°–5° C., and there is a total absence of light. It was hardly expected that animals would inhabit this region, until Forel discovered Asellus aquaticus in a depth of forty metres in the Lake of Geneva, and subsequently showed that quite a number of animals, including a Hydra, several worms, Molluscs, Crustacea, and larval Insects, may be found in these or even much greater depths.
The Crustacea of the abyssal region are two in number, and these have been found in a number of European lakes; Niphargus puteanus, a blind Amphipod closely allied to Gammarus; and Asellus forelii, allied to A. aquaticus and A. cavaticus, which may be either quite blind or else retain the rudiments of eyes.
These two Crustacea, under a practically identical form, are also found in the subterranean waters of Europe, and Forel considers that they have arrived in the abysses of the lakes from the subterranean channels, and are not derivatives of the littoral fauna.[168]
Having completed our short review of lacustrine Crustacea, we may deal with the subterranean and cave Crustacea,[169] which, as far as light and temperature are concerned, are subjected to very similar conditions to those dwelling at the bottom of deep lakes. The inhabitants of the subterranean waters have been chiefly brought to light in Artesian wells, etc., while the cavedwellers 210have been investigated especially at Carniola and in the American caves.
A number of species of Cyclopidae and Cypridae, many of which are blind and colourless, have been brought up in well-water. The Amphipod Niphargus puteanus has long been known from a similar source in England[170] and all over Europe, and several other blind Gammarids inhabit the subterranean waters and caves in various parts of the world. Among Isopods, Asellus cavaticus is recorded from wells and caves in various parts of Europe, Caecidotea stygia and C. nickajackensis from the Mammoth and Nickajack Caves in America, and two very remarkable blind Isopods are described by Chilton from the subterranean waters of New Zealand, viz. Cruregens fontanus, whose nearest allies are the marine Anthuridae, and the Isopods Phreatoicus typicus and P. assimilis, which bear an extraordinary resemblance superficially to Amphipods. Besides these, a small number of subterranean Decapoda are known which retain the eye-stalks but are without functional ommatidia. These are Troglocaris schmidtii, in Hungary, related to the fresh-water Atyid Xiphocaris of East Indian and East Asiatic fresh waters rather than to the South European Atyephyra; Palaemonetes antrorum, from artesian wells in Texas; and several species of Cambarus from the Eastern United States. A blind species of Cambarus, C. stygius, has been described from the caves of Carniola, and if this determination is correct, is the sole Cambarus occurring outside America.
It will be seen from the above account that the subterranean Crustacea are an exceedingly interesting and, in many respects, archaic group, many of which have survived in these isolated and probably uncompetitive districts, while many secular changes were going on in the quick world overhead.
The remaining fresh water Malacostraca may be mentioned under the headings of the groups to which they belong.
Only one “Schizopod,” apart from Paranaspides, is known from fresh-water lakes, viz. Mysis relicta, which was discovered in 1861 by Lovén in the Scandinavian lakes, and has since been found in the Finnish lakes, the Caspian Sea, Lake Michigan, and other localities in N. America, and Lough Erne in Ireland. This species is closely related to Mysis oculata of Greenlandic seas.
211In the Southern Hemisphere we have a species of Anaspides, A. tasmaniae, occurring in mountain streams and tarns in Tasmania, a related form which haunts the littoral zone of the Great Lake in Tasmania, and a small species, Koonunga cursor, occurs in a little stream near Melbourne.
Of the Isopoda certain genera, viz. Asellus and Monolistra, are confined to fresh water, others, such as Sphaeroma, Idothea, Alitropus, and Cymothoa, have occasional fresh-water representatives. Packard[171] describes a remarkable blind Isopod, Caecidotea, from the Mammoth Cave of Kentucky, which occupies a very isolated position, and in the same work gives a very complete exposition of the cave-fauna of North America and Europe.
The Phreatoicidae are a curious family of Isopods confined to the fresh waters of Australia and New Zealand, which bear a remarkable resemblance to Amphipods, being laterally compressed and possessing a subchelate hand on the anterior thoracic leg. These Isopods are exceedingly common in small mountain pools and in streams in Tasmania, and in the Great Lake in that country I have recently found a number of species which, together with some species of Amphipods, make up the dominant feature in the Crustacean fauna. One of these species may grow to fully an inch in length. The family is confined to the temperate regions, and is usually found on mountains. A number of species are known from the mainland of Australia, one coming from a high elevation on Mount Kosciusko, and another (Phreatoicopsis) from the forests of Gippsland attaining a great size, and living among damp leaves, etc.
The fresh-water Amphipoda all belong to the families Talitridae, Gammaridae, and Haustoriidae (see p. 137).
Among the Talitridae, or Sand-hoppers, Orchestia and Talitrus have marine as well as fresh-water and land representatives, while the American Hyalella is entirely from fresh water, most of the species being peculiar to Lake Titicaca. Many of these animals are partly emancipated from an aquatic life. Thus Orchestia gammarellus, which is common on the sea-shore of the Mediterranean, frequently penetrates far inland, and was found in large numbers by Kotschy near a spring 4000 feet up on Mount Olympus.
212Talitrus sylvaticus is very common among fallen leaves and decaying timber in Tasmania and Southern Australia, many miles from the sea, and often at an elevation of several thousand feet.
Among the Gammaridae, certain genera, e.g. Macrohectopus (Constantia), from Lake Baikal, are purely fresh-water. An enormous development of Gammaridae was discovered by Dybowsky in Lake Baikal, comprising 116 species, and lately a number more have been found by Korotneff.[172] The majority of these were originally placed in the genus Gammarus, but Stebbing has rightly created a number of peculiar genera for them. Certain species are, however, placed in more widely distributed genera, e.g. Gammarus and Carinogammarus, which is also represented in the Caspian Sea. Korotneff found some remarkable transparent pelagic forms (Constantia) swimming in the abyssal regions at about 600 metres depth, the majority of them being blind, but some possessing rudimentary eyes, often on one side only.
Besides various species of Gammarus, a number of other Gammaridae are frequently found in brackish water. Among Haustoriidae Pontoporeia has representatives in both the oceans and inland lakes of the northern hemispheres (see p. 137).
Of the Decapoda, seven families are typically fresh-water in habitat—the Aegleidae, containing the single species Aeglea laevis, related to the Galatheidae, which inhabits streams in temperate S. America; the Atyidae, a family of Prawns from the tropical rivers and lakes of the New and Old World, and in the Mediterranean region. A number of Palaemonidae are found in fresh water, e.g. Palaemonetes varians in Europe and N. America, while several species of Palaemon occur in lakes, streams, and estuaries of the tropical Old and New World.
The expeditions of Moore and Cunnington to Lake Tanganyika brought back an exceedingly rich collection of Prawns, comprising twelve species, all of which are peculiar to the lake,[173] and this is all the more surprising since Lakes Nyasa and Victoria Nyanza are only known to contain one species, Caridina nilotica, which ranges all over Africa and into Queensland and New Caledonia. The Tanganyika species, however, all belong to purely fresh-water genera, and do not afford any suggestion 213that they are part of a relict marine fauna. It would appear that they have been differentiated in the lake itself during a long period of isolation.
Two groups of Brachyura, viz. the Thelphusidae and the Sesarminae (a sub-family of the Grapsidae), are fresh-water. Thelphusa fluviatilis is an inhabitant of North Africa, and penetrates into the temperate regions of the Mediterranean, and is said to be exceedingly common in the Alban Lake near Rome. Both these families have representatives on land, e.g. Potamocarcinus in Central and South America, and certain species of Sesarma, and the closely related Gecarcinidae of the West Indies.
The remaining families to be dealt with are the two Crayfish families—the Astacidae and the Parastacidae—which live in rapidly moving rivers and streams, and occasionally in lakes. A few species of both families have taken to a subterranean mode of life, and excavate burrows in the earth, e.g. the Tasmanian Crayfish, Engaeus fossor. The distribution of the Crayfishes has long engaged the attention of naturalists. It was first seriously studied by Huxley,[174] and has subsequently been followed up, especially in North America, by Faxon[175] and Ortmann,[176] but our knowledge of the South American and Australian forms is still very incomplete. The Astacidae inhabit the northern temperate hemisphere, the Parastacidae the southern temperate hemisphere, the tropical belt being practically destitute of Crayfishes. Of the Astacidae the genus Astacus (Potamobius), including the common Crayfishes of Europe, occurs in Europe and in North America west of the Rockies. The genus Cambaroides, which in certain respects approaches Cambarus, is found in Japan and Eastern Asia. The very large genus Cambarus, on the other hand, only occurs in North America east of the Rockies, so that Cambaroides occupies a very isolated position. The occurrence of a Cambarus, C. stygius, in the caves of Carniola, has been recorded by Joseph, so that it would appear that this genus had a much wider range formerly than now.
Of the southern temperate Parastacidae, Australia and Tasmania have the genera Astacopsis and Engaeus; New Zealand 214has Paranephrops, South America Parastacus, and Madagascar Astacoides. The last named genus is rather isolated in its characters, possessing a truncated rostrum and a highly modified branchial system, but it agrees with all the other Parastacine genera, and differs from the Astacidae in the absence of sexual appendages on the first abdominal segment, and in the absence of a distinct lamina on the podobranchiae. The largest crayfish in the world is Astacopsis franklinii, found in quite small streams on the north and west coast of Tasmania. Specimens have been caught weighing eight or nine pounds, and rivalling the European Lobster in size. Crayfishes appear to be entirely absent from Africa.
It seems reasonable to suppose that the two families of Crayfishes characteristic respectively of the northern and southern hemispheres have been independently derived from marine ancestors, which have subsequently become extinct. Their complete absence in the tropics is striking, and Huxley drew attention to the fact that it is exactly in those regions where the Crayfishes are absent that the other large fresh-water Malacostraca are particularly well developed, and vice versa. Thus the large fresh-water Prawns are typically circumtropical in distribution, while the South African rivers abound with River-crabs, which, in general, are found wherever Crayfishes do not occur.
A few of the more interesting features in connection with the distribution of fresh-water Crustacea have now been touched upon. With regard to the origin of this fauna, we can see that a number of the species are comparatively recent immigrants from the sea, working their way up the estuaries of rivers, a proceeding which can be observed to be taking place to-day in a district like the Broads of Norfolk. Others, again, but these are few, appear to be true relict marine animals left stranded in arms of the sea that have been cut off from the main ocean, and have been gradually converted into fresh-water lakes and seas. Such are, perhaps, Mysis relicta and the rich Gammarid fauna of Lake Baikal, a lake that, in the presence of Seals, Sponges, and other marine forms, has clearly retained some of the characters of the ocean from which it was derived. The majority of the fresh-water species, however, have probably been evolved in situ, and their origin from marine ancestors is lost in an obscure past. The Crustacean fauna of the Caspian 215Sea[177] shows us in an interesting manner the effects of isolation and changes in salinity, etc., on the inhabitants of a basin which once formed part of the ocean. The waters of the Caspian Sea are not fresh, but they are on the average about one-third as salt as that of the open ocean. The Crustacea, described by Sars, belong to undoubtedly marine groups, e.g. the Mysidae, Cumacea, and Amphipoda Crevettina, but the remarkable feature of these Caspian Crustacea is the great variety of peculiar species representing marine genera which are very poorly represented in the sea, thus indicating that the variety of the fauna is not due to a great variety of species having been shut up in the Caspian Sea to begin with, but rather that, after the separation from the sea, the isolated species began to vary and branch out in the most luxuriant way—whether from lack of competition or owing to the changing conditions of salinity it is difficult to say. As an example, the Cumacea of the Caspian Sea are ten in number, all belonging to peculiar genera related to Pseudocuma, except one species which is included in that genus. These Caspian forms make up the Family Pseudocumidae, which contains in addition only two marine forms of the genus Pseudocuma (see p. 121). A very similar condition is found in the numerous Amphipods of the Caspian Sea. Considering the enormous changes that must have taken place in the distribution of land and water even during Tertiary times, it is astonishing that the fresh waters of the world do not contain more species in common with the ocean, but it must be considered that the limited area and comparatively uniform conditions of fresh-water lakes and streams would only permit a limited number of these forms to survive which could most easily adapt themselves to the changed conditions. And these would in all probability be the littoral species that were in the habit of passing up into the brackish waters of estuaries and lagoons, so that the uniform and limited nature of the fresh-water fauna can be accounted for to a certain extent by this hypothesis.
We have seen in dealing with the marine Crustacea of the littoral zone that the chief condition determining their distribution is temperature, and that the world may be divided into three chief 216areas of distribution for these animals, viz. the north temperate hemisphere, the tropics, and the south temperate hemisphere. It seems that the same division holds good for fresh-water Crustacea. We have already seen that the Crayfishes follow this rule, being practically absent from the tropics, and represented in the two temperate hemispheres by two distinct families, the Astacidae in the north and the Parastacidae in the south. Characteristic of the tropical belt are the absence of Crayfishes and the great development of Prawns and River-crabs. In the case of Entomostraca the great majority of the genera are cosmopolitan, especially those which live in small bodies of water liable to dry up, because these forms always have special means of dissemination in the shape of resting eggs which can be transported in a dry state by water-birds and other agencies to great distances; but those genera which inhabit large lakes are more confined in their distribution. The Copepod genus Diaptomus, characteristic of lake-plankton, ranges all over the northern hemisphere and into the tropics, but it is almost entirely replaced in the southern hemisphere by the related but distinct genus Boeckella,[178] which occurs in temperate South America, New Zealand, and southern Australia, and was found by the author to be the chief inhabitant in the highland lakes and tarns of Tasmania, Diaptomus being entirely absent. Of the Cladocera there are a number of pelagic genera (e.g. Leptodora, Holopedium, Bythotrephes) entirely confined to the lakes of the northern hemisphere. The distribution of Bosmina is interesting. This genus is distributed all over the north temperate hemisphere in lakes and ponds of considerable size, not liable to desiccation; in the New World it passes right through the tropics into Patagonia,[179] the chain of the Andes doubtless permitting its migration. In the tropics of the Old World it is unknown, but it turns up again, as the author recently found, as a common constituent in the plankton of the Tasmanian lakes. There is another instance of a group of Crustacea, characteristic of the north temperate hemisphere, being entirely absent from the tropics, at any rate of the Old World, but reappearing in the temperate regions of Australasia. The commonest fresh-water Amphipods in this region belong to the genus Neoniphargus, intermediate in its characters between the 217northern Niphargus and Gammarus, but grading almost completely into the latter. Both Niphargus and Gammarus are absolutely unknown from the tropics, but whether, like Bosmina, they occur in the Andes and temperate South America is not known; it seems, however, probable that they have reached Southern Australia by way of South America rather than through the tropics of Asia and Australia, where there is no range of mountains to permit the migration of a group of animals apparently dependent on a temperate climate. The other common fresh-water Amphipod in temperate Australia and New Zealand is Chiltonia, whose nearest ally is Hyalella from Lake Titicaca on the Andes, and temperate South America.
The Anaspidacea and Phreatoicidae, which are so characteristic of temperate Australia, and are generally of an Alpine habit, have never been found in South America, but the Anaspidacea are represented by numerous marine forms in the Permian and Carboniferous strata of the northern hemisphere, so that it is probable that this group reached the southern hemisphere from the north through America.
The distribution of the fresh-water Crustacea, therefore, in the temperate southern hemisphere affords strong evidence in favour of the view that the chief land-masses of this hemisphere, which are at present separated by such vast stretches of deep ocean, were at no very remote epoch connected in such a way as to permit of an intermixture of the temperate fauna of New Zealand, Australia, and South America. While this connexion existed, a certain number of forms characteristic of the northern hemisphere, which had worked through the tropics by means of the Andes, were enabled to reach temperate Australia and New Zealand. The existence of a coast-line connecting the various isolated parts of the southern hemisphere would, of course, also account for the community which exists between their littoral marine fauna. It is impossible to enter here into the nature of this land-connexion which is becoming more and more a necessary hypothesis for the student of geographical distribution, whatever group of animals he may choose, but it may be remarked that the connexion was probably by means of rays of land passing up from an Antarctic continent to join the southernmost projections of Tierra del Fuego, Tasmania, and New Zealand.
TRILOBITA
CHAPTER VIII
TRILOBITA
Among the many interesting groups of fossils found in the Palaeozoic deposits there is none which has attracted more attention than the Trilobites. As early as 1698, Edward Lhwyd, Curator of the Ashmolean Museum in Oxford, recorded in the Philosophical Transactions the discovery of Trilobites in the neighbourhood of Llandeilo in South Wales; and of one of his specimens he remarked that “it must be the Sceleton of a flat Fish.” In the following year the same writer gave in his Lithophylacii Britannici Ichnographia descriptions and figures of two Trilobites which are evidently examples of the species now known as Ogygia buchi and Trinucleus fimbriatus.
Although Trilobites differ so much from living Arthropods that it was difficult to determine even whether they belonged to the Crustacea or the Arachnida, yet one of the earliest writers, Dr. Cromwell Mortimer, Secretary of the Royal Society (1753), recognised their resemblance to Apus (see pp. 19–36). This view of their affinities was adopted by Linnaeus, and has been supported by many later writers. Another early author, Emanuel Mendez da Costa, thought that the Trilobites were related to the Isopods, an opinion which has been held by some few zoologists of more recent times.
The Trilobites form the only known Order of the Crustacea which has no living representatives. They are found in the oldest known fossiliferous deposits—the Lower Cambrian or Olenellus beds, where they are represented by 19 genera belonging to the families Agnostidae, Paradoxidae, Olenidae, and Conocephalidae. From the variety of forms found and the state of development which they have reached, it is evident that even at that remote 222period the group must have been of considerable antiquity; but of its pre-Cambrian ancestors nothing is yet known; consequently there is no direct evidence of the origin of the group.
Trilobites form an important part of all the faunas of the Cambrian system; they attain their greatest development in the Ordovician period, after which they become less numerous; their decline is very marked in the Devonian, in which nearly all the genera are but survivals from the Silurian period; in the Carboniferous, evidence of approaching extinction is seen in the small number of genera represented, all of which belong to one family—the Proëtidae, in the relatively few species in each genus and in the small size of the individuals of those species. In Europe no representatives of the group appear to have survived the Carboniferous period, but in America one form has been recorded from deposits of Permian age.
Trilobites seem to have been exclusively marine, since they are found only in association with the remains of marine animals. Their range in depth was evidently considerable, for they occur in many different kinds of sediment, and were apparently able to live regardless of the nature of the sea-floor—whether muddy, sandy, calcareous, or rocky. In some cases they occur in deposits containing reef-building corals and other shallow water animals; in others they are associated with organisms which lived at greater depths. The group appears to have had a world-wide distribution, for the remains of Trilobites are found in the Palaeozoic rocks of all countries. Their range in size is considerable; for whilst a large proportion of the species are about two or three inches in length, some, like Agnostus, are only a quarter of an inch long, others are from ten to twenty inches long, the largest forms including species of Paradoxides, Asaphus, Megalaspis, Lichas, and Homalonotus.
The feature in a Trilobite which first attracts attention is the marked division of the dorso-ventrally flattened body into a median or axial part, and a lateral or pleural part on each side. It was this character that led Walch, in 1771, to give the name by which the group is now known. The axial part of the body contained the alimentary canal, as is shown by the position of the mouth and anus, as well as by casts in mud of the canal which are found in some specimens. The trilobation of the 223body is quite distinct in the majority of Trilobites, but in a few genera belonging to the Asaphidae and Calymenidae (Fig. 136) it becomes more or less completely obsolete.

Fig. 136.—Homalonotus delphinocephalus, Green, × 1. Silurian. (After Zittel.)
In most cases the only part of the Trilobite which is preserved is the exoskeleton which covered the dorsal surface of the body. That skeleton consists largely of calcareous material, and shows in sections a finely perforated structure. Generally it is arched above, but in some cases is only slightly convex; in outline it is more or less oval. Three regions can always be distinguished in the body of a Trilobite—the head, the thorax, and the abdomen or pygidium.
The carapace which covers the head is known as the cephalic shield (Fig. 137, A, 1), and is commonly more or less semicircular in outline, but varies considerably in different genera. Only in a few cases, as in some species of Agnostus (Fig. 146), is its length greater than its breadth. The axial part of the cephalic shield, called the “glabella” (Fig. 137, A, a), is usually more convex than the lateral parts (“cheeks” or “genae”), and is separated from them by longitudinal or axial furrows (b). The shape of the glabella varies greatly; it may be oblong, circular, semi-cylindrical, pyriform, spherical, etc. Its relative size likewise varies; thus in Phacops cephalotes it expands in front and forms the larger part of the head, whilst in Arethusina (Fig. 151, B) it is narrow and short, being only about one-half of the length of the head.

Fig. 137.—Calymene tuberculata, Brünn. × 1. Silurian, Dudley. A, Dorsal surface: 1, head; 2, thorax; 3, pygidium or abdomen. a, Glabella; b, axial furrow; c, glabella-furrow; d, neck-furrow; e, fixed cheek; f, free cheek; g, facial suture; h, eye; i, genal angle; k, axis of thorax; l, pleura. B, Ventral surface of head (after Barrande): a, hypostome; b, doublure; c, c′, facial sutures; d, rostral suture; e, rostral plate. C, One segment of the thorax: a, ring of axis; b, groove; c, articular portion; d, axial furrow; d-f, pleura; d-e, internal part of pleura; e-f, external part of pleura; e, fulcrum; g, groove. D, Coiled specimen: a, glabella; b, eye; c, facial suture; d, pygidium; e, rostral suture; f, continuation of facial suture.
The segmentation of the head is indicated by transverse furrows on the glabella (Fig. 137, A, c, d). In some cases these furrows extend quite across the glabella (Fig. 147), but commonly they are found on the sides only and divide the glabella into lateral lobes. Only the posterior or “neck-furrow” (Fig. 137, A, d) is continued on to the cheeks, and the segment which it limits anteriorly on the glabella[180] is known as the occipital or neck-ring. In front of the neck-furrow there may be three other furrows, so that altogether five cephalic segments are indicated by the furrows of the glabella. Commonly all the furrows are distinct in the primitive types; but in the more modified forms some, especially the anterior, become either reduced in size or obsolete. The actual number of furrows present consequently varies in different genera, and may even differ in different species of the same genus. In a few genera all the furrows are either indistinct or absent, as for example in Ellipsocephalus (Fig. 150, B). In some cases four furrows are present in addition to the neck-furrow; this is due to the division of the 225anterior lobe of the glabella by fulcra which are developed for the attachment of muscles.
When the glabella reaches the front border of the head the two cheeks are separated (Fig. 150, I); but in other cases they unite in front of the glabella (Fig. 150, C). The outer posterior angle of the cheeks or genae (“genal angle,” Fig. 137, A, i) may be rounded, pointed, or produced into backwardly directed spines (Fig. 140). The marginal part of the cephalic shield is often flattened or concave; this border may be quite a narrow rim as in Calymene (Fig. 137, A), but in some genera (e.g. Trinucleus, Fig. 140, B; Harpes, Fig. 150, A; Asaphus) it attains a great development. Each cheek is usually divided by a suture—the “facial suture” (Fig. 137, A, g)—into an inner and an outer part; the former is the “fixed cheek” (e), and the latter the “free cheek” (f). The course of the facial suture varies in different genera: on the posterior part of the head it begins either at the posterior margin (Fig. 150, C) or at the posterior part of the lateral margin (Fig. 151, C, D); at first it is directed inwards, and then bends forward, forming an angle. In front it may (a) end at the front margin (Fig. 147), or (b) be united beneath the front margin by a rostral suture (Fig. 137, B, d, D, e), or (c) unite with the other suture on the dorsal surface in front of the glabella (Fig. 151, C). In the last case the free cheeks also unite in front of the glabella.
The facial suture is one of the distinguishing features of the Trilobites, and may have been of some use in ecdysis. In only a few forms is it absent, as for example in Agnostus (Fig. 146) and Microdiscus. In the former, however, Beecher states that a suture is really present, but, unlike that of most other Trilobites, it is situated at the margin of the cephalic shield, and consequently the free cheek, if present, must be on the ventral surface. Lindström and Holm, after a re-examination of well-preserved specimens, deny the existence of a suture in Agnostus. By most authors Olenellus is said to be without a suture, but Beecher maintains that although the fixed and free cheeks have coalesced, yet a raised line passing from the eye-lobe to the posterior margin marks the position of the suture; this view is not accepted by Lindström.
The existence of a facial suture in Trinucleus has likewise 226been disputed. But Emmerich, Salter, and M‘Coy[181] have maintained that a suture is present in a normal position on the dorsal surface, extending from the posterior margin just within the genal angle to the eye (when present), and from thence bending forward and ending on the front margin near the glabella. It must be admitted that no indications of the suture are seen in the majority of specimens, perhaps owing to the fact that most examples of Trinucleus are in the form of internal casts; perhaps also to the more or less complete coalescence of the fixed and free cheeks, since in no specimen has the free cheek been found separated from the rest of the head, as occurs not uncommonly in many other Trilobites. The probability of the existence of a suture receives some support from the fact that one is found in the allied genera Orometopus and Ampyx (Fig. 140). Barrande and Oehlert deny its existence in Trinucleus. There is, however, in that genus a suture running close to the margin of the cephalic border,[182] and joining the genal angle so as to cut off the genal spine. Lovén and Oehlert claim that this suture represents the facial suture, but in an abnormal position; this view, however, is not accepted by Beyrich. In this connection it should be noted that in Acidaspis, whilst the majority of the species possess a facial suture, there are two in which it has disappeared owing to the fusion of the fixed and free cheeks. Such being the case, it seems not improbable that the curved line passing backwards from the eye in Harpes may mark the position of the suture; but it is stated that the only suture present in that form runs at the margin of the cephalic border, and is similar to that of Trinucleus. This matter will be referred to again when discussing the nature of the eyes in Trinucleus and Harpes.
The relative sizes of the fixed and free cheeks obviously depend on the position of the facial suture; when this starts on the lateral margin of the cephalic shield and passes forward to the outer part of the front margin, the free cheek will be a narrow strip; when, on the other hand, the suture starts from the posterior margin and runs close to the glabella, the free cheek will be relatively large and the fixed cheek narrow. The 227fixed cheek is small in Phacops, Cheirurus, and Illaenus; relatively large in Remopleurides, Phillipsia, and Stygina. It was suggested by M‘Coy[183] that the free cheek represents the pleura of an anterior segment which has not become fused with the other cephalic pleurae. The fixed cheek appears to be formed of the coalesced pleurae of the other cephalic segments, but of those pleurae the only indication seen in adult specimens is in the neck-ring; in young specimens of Olenellus, however, the presence of other pleurae is indicated by furrows on the cheeks in front of the neck-furrow.

Fig. 138.—Phacops latifrons, Bronn, × 1. Devonian. Showing large compound eye. (After Zittel.)
A pair of compound eyes are present in the majority of Trilobites. Each eye is situated on the free cheek, at that part of its inner margin where the facial suture bends to form an angle (Figs. 137, A, h, 138). The position of the eye is consequently determined by the position of the facial suture; it may be near the glabella or near the lateral margin of the head, and either as far forward as the first segment of the glabella or nearly as far back as the neck-furrow. In many Trilobites the eye is more or less conical, with its summit truncated or rounded, but in some genera it is ovoid, or crescentic. In Aeglina (Fig. 150, H) the eye is flattened and scarcely raised above the general level of the cheek. The eye of a Trilobite is oriented so that its longer axis is parallel or nearly parallel to the axis of the body (Fig. 150, G); but in one case (Encrinurus intercostatus) it is placed at right angles to this axis. The size of the eye varies considerably; it is largest in Aeglina, in which it covers nearly the whole of the free cheek; it is small in Acidaspis and Encrinurus.
Though the eye is always entirely on the free cheek, the adjoining part of the fixed cheek is raised to form a buttress on which the eye rests; this buttress, which is known as the “palpebral lobe,” is seen clearly when the fixed cheek is removed. The eyes of Trilobites are always sessile; for although in some species, such as Asaphus cornigerus, A. kowalewskii, and Encrinurus punctatus, they are on the summits of prominent stalks, yet those stalks are immovable.
228Three types of compound eye have been recognised in Trilobites[184]—holochroal, prismatic, and schizochroal.

Fig. 139.—Eyes of Trilobites. (After Lindström.) A, B, Sphaerophthalmus alatus, Ang. Upper Cambrian. Vertical and horizontal sections, × 100. C, Asaphus fallax, Dalm. Horizontal section, × 60. D, Nileus armadillo, Dalm. Vertical section, × 60, a, prismatic lenses; b, cuticle; c, part of free cheek. E, Dalmanites vulgaris, Salt. Part of eye, × 30. F, Dalmanites imbricatulus, Ang. Vertical section of eye, with a part of the free cheek on the left, × 60. G, H, Harpes vittatus, Barr. G, The two lenses of one eye, × 8; H, vertical section of the same, × 60.
1. In the holochroal eye (Fig. 139, A, B) the lenses are globular or biconvex and close together, so that the cornea is continuous over the entire eye. Examples of this are seen in Bronteus and Sphaerophthalmus.
2292. In the prismatic type (Fig. 139, C, D) the lenses are prismatic and plano-convex, and the entire surface of the eye is covered by a smooth cuticle. The lenses are close together and usually hexagonal, but occasionally rhombic or square. Near the margin of the eye the lenses may become irregular, giving rise to a border in which the prismatic structure is more or less indistinct. The prismatic type of eye is found in the genera Asaphus, Nileus, Illaenus, etc.
3. The schizochroal eye occurs only in the family Phacopidae (Fig. 139, E, F). The lenses are biconvex and are separated by portions of the cephalic shield, so that each lens appears to rest in a separate socket, and the cornea is not continuous for the entire eye. The lenses are circular in outline, but owing to the upward and inward growth of the interstitial test they may appear, on the surface, to be hexagonal. The diameter of a lens may be as much as 0·5 mm. The crystalline cones have not been preserved. In specimens of Phacops rana, in which the inner face of the lens is more convex than the outer, J. M. Clarke[185] has obtained evidence of a posterior spheroidal cavity in addition to the anterior corneal cavity. The complete separation of the lenses in this type of eye has led to the suggestion that the schizochroal eye is an aggregate rather than a compound eye. But the difference between this and the holochroal eye is probably less than appears at first sight if the statement made by Clarke is confirmed, namely, that in young specimens of Calymene senaria the lenses are relatively large and similar to those of Phacops, whereas in the adult the eye is holochroal.
These three types of eye, according to Lindström, have appeared successively in chronological order: the prismatic occurring first in the Olenus beds (Upper Cambrian), the holochroal first in the Ceratopyge Limestone (Uppermost Cambrian), and the schizochroal first in the Ordovician. The number of lenses in the eye varies greatly. For example, in Trimerocephalus volborthi there are 14 only, whilst in Remopleurides radians there are as many as 15,000. Even in different species of the same genus there may be considerable differences. Thus Bronteus brongniarti possesses 1000, B. palifer 4000, lenses in each eye. The number increases from the young up to the adult, but decreases in old age. The lenses are usually arranged in 230alternating rows. In Trilobites with a conical eye the outer segment of the cone bears the visual surface. It has been stated that the eyes of Trilobites resemble those of Isopods,[186] but close comparison is difficult to make, since in Trilobites no part of the eye beneath the lenses is preserved. In some genera a threadlike ridge, called the “eye-line,” passes from the glabella, generally from the front segment, to the eye, where it often ends in the palpebral lobe; this eye-line is found in nearly all genera which are confined to the Cambrian period, and persists in a few of later date, as for example in Triarthrus, Euloma, and some species of Calymene from the Ordovician; in Arethusina and Acidaspis from the Silurian; and in Harpes from the Devonian (Fig. 150, A).

Fig. 140.—Trinucleidae. A, Orometopus elatifrons, Ang. × 5. Restoration based on specimens from the Upper Cambrian (Tremadoc) of Shineton, Shropshire. B, Trinucleus bucklandi, Barr. Ordovician, Bohemia. A complete but not fully-grown individual showing eyes. Natural size. (After Barrande.) C, Ampyx rouaulti, Barr. × 3. Ordovician, Bohemia. (After Barrande.)
In Harpes and in some species of Trinucleus eyes are present, but have been stated to be of a different type. They are described as simple eyes, and have been compared with ocelli; they are never found in Trilobites which possess the compound eyes described above. In Harpes (Fig. 150, A) the eye is near the middle of the cheek, in the position where compound eyes occur in other genera; it appears to consist of two or three granules or tubercles which are really lenses, and is connected with the front of the glabella by an eye-line. No facial suture can be seen, consequently the whole of the cheek is stated to be the fixed cheek.[187] In Trinucleus (Fig. 140, B) a single tubercle is 231found on the middle of the cheek in the young of some species, and is sometimes connected with the glabella by an eye-line; the latter disappears before the adult state is reached, and in some species the tubercle also disappears, but in others (such as T. seticornis, T. bucklandi) it persists in the adult individuals.
From the lateral position of these eyes they can hardly be compared with the median simple eye of other Crustacea. In Harpes it is more probable that, as suggested by J. M. Clarke, they are schizochroal eyes imperfectly developed. Their structure (Fig. 139, G, H) is somewhat similar to that of schizochroal eyes, and moreover, in one species, H. macrocephalus,[188] there are, in addition to the three main tubercles, other smaller tubercles in regular rows. Further, the eye-line occupies the same position as in other Trilobites which have undoubted compound eyes. The absence of a facial suture cannot be taken as evidence against these eyes being of the ordinary type, since in some species of Acidaspis (e.g. A. verneuili, A. vesiculosa) which possess compound eyes there is, in consequence of the coalescence of the fixed and free cheeks, no suture.
In some species of Trinucleus (Fig. 140, B) the simple eye is found in the same position as the eye in Harpes, and if, as some writers have maintained, there is evidence of the existence of a suture in that genus, then there is no reason for regarding the eye as other than a degenerate form of compound eye. The probability of its being such is supported by the existence of a compound eye in a similar position in the allied form Orometopus (Fig. 140, A) which possesses a facial suture.
In some species of Trinucleus (Fig. 140, B) and Ampyx there is a small median tubercle on the front part of the glabella, which from its position may be a simple unpaired eye, but its structure appears to be unknown.
Some Trilobites possess no eyes. Well-known examples of such are Agnostus, Microdiscus, Ampyx, Conocoryphe, and some species of Illaenus and Trinucleus; such blind Trilobites are almost confined to the Cambrian and Ordovician periods. All the forms of later periods, with the exception of a species of Ampyx, and possibly one or two other species, possess eyes. In addition to those undoubtedly blind forms Lindström considers that most of the Olenidae and Paradoxidae were without eyes. 232Many of the members of these families possess a lobe closely resembling a palpebral lobe, and a corresponding excavation in the free cheek; such forms have been generally regarded as possessing eyes; and the absence of any indication of lenses in those cases, on which Lindström lays stress, has been explained by the comparatively imperfect preservation of these early Trilobites. The development of the supposed eye-lobe in some of the Paradoxidae and Olenidae differs from that of the eyes in other families of Trilobites. In the latter the eye appears first at the margin of the head and always in connexion with the facial suture. But in Olenellus, in which there is said to be no facial suture, development shows that the crescentic eye-like lobe (Fig. 145, E) is really of the nature of a pleura coming off from the base of the first segment of the glabella. In Paradoxides, which resembles Olenellus in many respects, a facial suture is present and forms the outer boundary of the eye-like lobe, but it is developed subsequently to the appearance of the latter, which seems to be similar to that of Olenellus. In some genera of the Olenidae the eye-line, which comes off from the first segment of the glabella, ends in some cases in a swelling or knob which has hitherto been regarded as a palpebral lobe, but according to Lindström’s view no trace of an eye has been found in connexion with that lobe, nor is there any space between the lobe and the free cheek in which the eye could have occurred. If this view is correct it follows that the majority of the Cambrian Trilobites were blind. The earliest genus with eyes would then be Eurycare found in the Olenus beds of the Upper Cambrian. Sphaerophthalmus and Ctenopyge, found in the higher beds of the Cambrian, also possessed eyes, but Olenus and Parabolina were probably blind.
On the ventral surface of the head there is a flat rim around the margin (Fig. 137, B, b); this rim or “doublure” is the reflexed border of the cephalic shield. In many Trilobites its median part in front is cut off by sutures so as to form a separate plate (e); such is the case when the two facial sutures (c, c′) cut the anterior margin of the cephalic shield and are continued across the doublure, where they are joined by a transverse or rostral suture (d) just below the margin. When, however, as in Phacops and Remopleurides, the two facial sutures unite on the dorsal surface, in front of the glabella, the median part of 233the doublure is not separated from the lateral parts, or from the dorsal part of the cephalic shield.

Fig. 141.—A, Hypostome of Bronteus polyactin, Ang. showing maculae, × 4. B, Left macula of Bronteus irradians, Lindst. × 12. (After Lindström.)
The “labrum” or “hypostome” is attached to the doublure in front (Fig. 137, B, a); it is commonly an oval or shield-shaped plate, but is occasionally nearly square. Its surface is sometimes divided into two or three areas by shallow transverse grooves (Fig. 141, A), Just behind the middle of the hypostome, or when transverse grooves are present either in or near the anterior groove, there are often found a pair of small patches or “maculae” which are more or less oval or elliptical in outline (Fig. 141). The maculae may be (1) surrounded by a raised border, or (2) in the form of pits, or (3) raised like tubercles. In some cases the entire surface of a macula is smooth and glossy; in others either the whole or a part is covered with granules, and in the latter case the granules may be limited to the internal third (Fig. 141, B) or to the central portion. Sections of a macula show that the granules are really globular lenses similar to those of the compound eyes on the dorsal surface of the head. Some of the maculae which are without lenses show no structure, but in others there is a spongy or irregularly polyhedric structure with prisms, resembling the marginal zone of the prismatic eyes of some genera. There seems no doubt that the maculae with lenses are visual organs, and those without are degenerate eyes. They occur in some genera which, according to Lindström, are without eyes on the dorsal surface. Maculae do not appear to be present in other Crustacea, but they have been compared with a median organ, found just in front of the hypostome in Branchipus.[189] Maculae, 234have so far been found in 136 species of Trilobites belonging to 39 genera ranging from Lower Cambrian to Carboniferous.
A “metastoma” or lower lip plate (Fig. 142, Ep) is found just behind the hypostome in Triarthrus, but has not been noticed in any other genus. Between the hypostome and the metastoma lies the mouth.
The segments of the thorax are free, and their number varies from two in Agnostus (Fig. 146) to twenty-six in Harpes (Fig. 150, A). In the Trilobites confined to the Cambrian period the number (except in the Agnostidae) is usually larger than in the genera found in the Ordovician and later periods. Owing to the free thoracic segments many Trilobites were able to curl up somewhat after the manner of a Wood-louse (Figs. 137, D, 138). The axial part of each thoracic segment is more or less considerably arched. Usually it consists of three parts: (i.) the largest part (Fig. 137, C, a), called the ring, is band-like in form, and is always visible whether the Trilobite is extended or coiled up; (ii.) in front of the ring is a depressed, groove-like part (Fig. 137, C, b) separating it from (iii.) the articular portion (c) which is convex in front and extends beneath the ring of the preceding segment; this part is only visible when the Trilobite is coiled up or when the segments are separated. In some few genera the axial part consists of a simple arched band without either a groove or a specially modified articular portion. The pleurae (Fig. 137, A, l, C, d-f) are fixed firmly to the axis, and have the form of narrow bands with the ends rounded, obtuse, pointed, or spinose. In a few cases the pleurae have a plain surface; but usually they possess either a ridge or a groove (Fig. 137, C, g); the former is generally parallel to the margins of the pleura, the latter is generally oblique, being inclined backwards from the axis. Sometimes in front of the ridge there is a small groove. On the ventral surface each pleura shows, at its outer extremity, a reflexed margin or doublure. At some distance from the axis the pleurae are bent downwards and backwards. The point where this bend occurs is called the “fulcrum” (e); it divides the pleura into an internal and an external part: the internal part (d-e) is flat or slightly convex, and just touches the front and back margins of the adjacent pleurae; the external part (e-f) may be (i.) narrower than the internal part, so that it is separated from the previous and 235succeeding pleurae; such occurs principally in pleurae with ridges, as in Cheirurus and Bronteus; or (ii.) it may be in the form of a long cylindrical process, as in many species of Acidaspis; or (iii.) the external part may be of the same width, either throughout or in part, as the internal part, and may overlap the next pleura behind; this type is found principally in pleurae with a groove such as in Phacops, Calymene, Sao, Asaphus, Ellipsocephalus.
In some Trilobites there is beyond the fulcrum a smooth, flat, triangular part at the front margin of the pleura; this part is known as the “facet,” and forms a surface articulating with the preceding segment which overlaps it.
In the remarkable form Deiphon (Fig. 151, E) the pleurae are separate throughout their entire length.
In some Trilobites broad and narrow forms of the same species occur—the difference being seen especially in the axis. The former are regarded as females, the latter as males.[190]
The segments of the abdomen or pygidium (Fig. 137, A, 3) are similar to those of the thorax, except that they are fused together. In a few forms, such as Illaenus (Fig. 150, F) and Bumastus, the fusion is so complete that no trace of segmentation can be seen on the dorsal surface. Usually, however, the segments are easily distinguishable; the number seen on the axis is commonly greater than on the lateral parts of the pygidium; this difference is particularly well shown in Encrinurus. In Trilobites which have grooved pleurae the conspicuous grooves seen on the lateral parts of the pygidium are the grooves of the pleurae, the sutures between the pleurae being less distinct. The shape of the pygidium may be semicircular, a segment of a circle, trapezoidal, triangular, semi-parabolic, etc.; its size varies considerably; in the Cambrian forms it is usually small, but in the Trilobites of later periods it becomes relatively larger. The number of segments in the pygidium varies from two to twenty-eight. The axis of the pygidium tapers more rapidly than that of the thorax; sometimes it reaches quite to the posterior end of the body, but is commonly shorter than the pygidium; in Bronteus it is extremely short, and the grooves on the lateral parts of the 236pygidium radiate from it in a fan-like manner. Occasionally, as in Bumastus, the axis cannot be distinguished from the lateral parts. In a few early Trilobites (Olenellus, Holmia, Fig. 148, Paradoxides, Fig. 147) the lateral parts of the pygidium are very small. In some genera, such as Asaphus, the marginal part of the pygidium forms a flattened or concave border. The margin may be entire or produced into spines, and sometimes (Fig. 151, C) a caudal spine comes off from the end of the axis. On the ventral surface of the pygidium there is a marginal rim similar to the doublure of the cephalic shield. The anus is on the ventral surface of the last segment of the pygidium.
Although Trilobites are often found in abundance and in an excellent state of preservation, it is only in very rare cases that anything is seen of the ventral surface except the hypostome and the reflexed borders of the cephalic shield, of the thoracic segments, and of the pygidium. The usual absence of appendages is probably due to their tenuity. Billings, in 1870, first obtained clear evidence of the presence of pairs of appendages, in Asaphus platycephalus. Soon afterwards Walcott[191] showed their existence in American specimens of Asaphus megistos, Calymene senaria, and Cheirurus pleurexacanthus. In the two latter species the appendages were found by cutting sections of curled-up specimens obtained from the Trenton Limestone; 2200 examples were sliced, of which 270 showed evidence of the existence of appendages. They were seen to be present on the head, thorax, and pygidium; a ventral uncalcified cuticle with transverse arches was also found. By means of sections of curled-up specimens it was difficult to determine satisfactorily the form and position of the appendages. Subsequently extended specimens of Triarthrus (Fig. 142) and Trinucleus, showing the ventral surface and appendages clearly, were discovered in the Utica Slate (Ordovician) near Rome, New York. A full account of the appendages in those specimens has been given by Beecher.[192]

Fig. 142.—Triarthrus becki, Green, × 2½. Utica Slate (Ordovician), near Rome, New York. A, Ventral surface with appendages; Ep, metastome; Hy, hypostome. B, second thoracic appendage; en, endopodite; ex, exopodite, × 12. (After Beecher.)
In Triarthrus each segment, except the anal, bears a pair of appendages, all of which, except the first, are biramous. There are five pairs of cephalic appendages; the first pair are attached at each side of the hypostome, and have the structure of antennae, 237each consisting of a single flagellum formed of short conical joints. The other cephalic appendages increase in size successively. At present the second and third pairs are not satisfactorily known, but appear to have been similar to the fourth and fifth pairs. The second pair is attached at the level of the posterior end of the hypostome. The fourth and fifth pairs have large, triangular coxopodites which served as gnathobases, their inner edges being denticulate; the endopodites consist of stout joints; the exopodites are slender, and bear setae which are often arranged in a fan-like manner.
The first pair of appendages appear to be antennules, whilst the second pair probably represent the antennae, the third pair the mandibles, and the fourth and fifth pairs the maxillae of other Crustacea. The appendages of the thorax and pygidium do not differ essentially from the two posterior cephalic appendages. Those on the anterior part of 238the thorax are the longest; the others gradually decrease in size in passing posteriorly. Each thoracic leg (Fig. 142, B) consists of a short coxopodite with an inward cylindrical prolongation forming a gnathobase which is best developed on the anterior legs; the endopodite and exopodite are long and nearly equal; the former consists of six joints tapering gradually to the end; the latter, of a long proximal joint with a denticulate edge and a distal part of ten or more joints, and it bears a row of setae along the whole of the posterior edge.
The anterior appendages of the pygidium differ but little from the posterior thoracic legs; but the phyllopodous character, which appears in the latter, becomes more distinct in the appendages of the pygidium, especially those near its posterior end, and is due to the broad, flat, laminar joints of the endopodite.
The more striking features of the appendages of Triarthrus are the small amount of differentiation which they show in different parts of the body, especially the want of specialisation in the cephalic region; the distinctly biramous character of all except the first pair; and the presence of one pair of functional antennae only, and the occurrence of thoracic gnathobases.
In Trinucleus the appendages are not so well known, but they are considerably shorter than in Triarthrus.
In the Palaeozoic rocks of Bohemia, where Trilobites are very perfectly preserved, Barrande[193] discovered the larval forms of several species, and in some cases was able to trace out the development very completely, but in others the earliest stages were not found. In the strata in which Trilobites occur Barrande found minute spheroidal bodies, usually of a black colour, and only a little smaller than the youngest larval stages; those bodies are probably the eggs of Trilobites. Since the publication of Barrande’s work the development of some species found in North America has been studied by Ford, Matthew, Walcott, and Beecher. But even now the development is known in only a very small proportion of the total number of genera of Trilobites. The early larval form (Fig. 143, A) is similar in general character in the various species in which it has been found. It is circular or ovoid in outline, with a length of from 0·4 to 1 mm., and consists of a large cephalic and a small pygidial portion; the axis is distinct and usually shows more or less clear 239indications of five cephalic segments; the eyes, when present, are found at or near the front margin, and the free cheeks, if visible at all on the dorsal surface, are narrow. For this early larval form Beecher has proposed the name “protaspis”; he regards it as the representative of the Nauplius of other Crustacea, but that view is not accepted by Professor J. S. Kingsley.[194]
The general changes which occur in the course of development are: modifications in the shape and relative size of the glabella, and of the number and depth of the glabella-furrows; the growth of the free cheeks and the consequent inward movement of the facial sutures and eyes; the introduction of and gradual increase in number of the thoracic segments, and the relative decrease in size of the head.

Fig. 143.—Development of Sao hirsuta, Barr. Cambrian. A, Protaspis; B-F, later stages; G, adult. The small outlines below each figure show the actual size of each specimen. (After Barrande.)
Sao hirsuta is a species found in the Cambrian, the development of which was fully described by Barrande. Its earliest protaspis stage (Fig. 143, A) is circular in outline; the glabella expands in front and reaches the anterior margin; the pygidial region is not distinctly separated from the cephalic region; segmentation is indicated in the former, and the neck-ring is present in the latter; the eye-line is seen on each side of the glabella near the anterior margin. In a later stage (Fig. 143, C) the segmentation of the glabella becomes more distinct, indicating the existence of five cephalic segments, and the facial suture appears near the margin limiting a very narrow free cheek. Subsequently (Fig. 143, D-F) the thoracic segments develop, and increase in number until the adult stage (G) is reached; also the eyes appear at the margin of the cephalic shield, and gradually move inwards, and the glabella becomes narrower and rounded in front, and ceases 240to reach the anterior margin. In this species the eye-line is present in the adult.
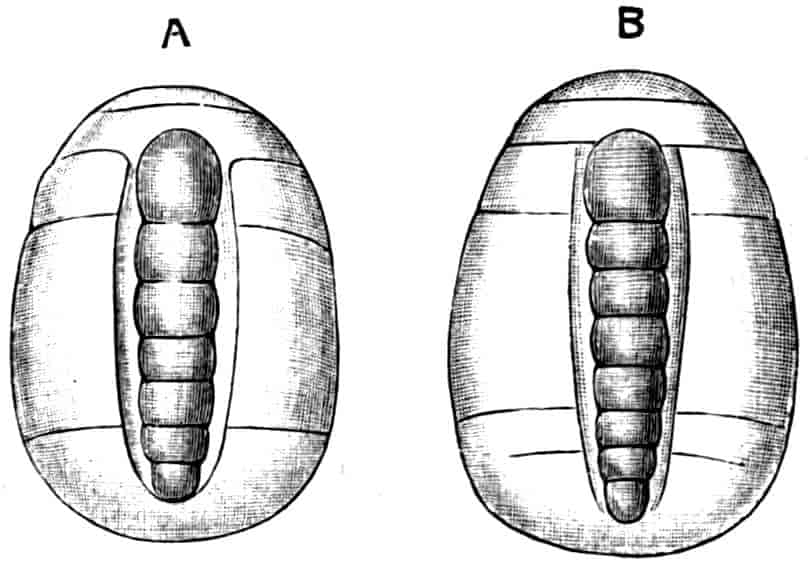
Fig. 144.—Triarthrus becki, Green. Ordovician. A, B, Two successive stages of the protaspis, × 45. (After Beecher.)
In the protaspis of Triarthrus (Fig. 144), found in the Ordovician, the glabella does not reach the front margin nor expand in front as it does in Sao; an eye-line is present, but disappears before the adult stage is reached.

Fig. 145.—Larval stages of Trilobites. A-D, Dalmanites socialis, Barr. Ordovician, Bohemia. The small figures below show the natural size of each specimen. (After Barrande.) E, Mesonacis asaphoides, Emmons, × 10. Lower Cambrian, North America. (After Walcott.) F, Acidaspis tuberculata, Conrad, × 20. Lower Helderberg Group (Lower Devonian or Upper Silurian), Albany County. (After Beecher.)
Dalmanites (Fig. 151, C) is a more advanced type than Sao and Triarthrus, and is found in later deposits. In the earliest stage (Fig. 145, A) the head and pygidium are quite distinct, and there is no eye-line present at this or any stage in development, but large ovoid eyes are found on the front margin, and have their long axes placed transversely to the axis of the body; the glabella is strongly segmented and is rounded in front. In later stages (C, D) the pygidium increases in size relatively, and the thoracic segments are successively introduced; the facial sutures and free cheeks appear 241on the dorsal surface, and as the free cheeks grow the eyes move inwards and backwards, and gradually swing round until their long axes become parallel with the axis of the body.
The larval form of Acidaspis (Fig. 145, F) is of interest since even in the earliest stage it shows the spiny character which forms such a striking feature of the adult (Fig. 151, F).
Before the discovery of the ventral surface of Trilobites it was thought by some zoologists that their affinities were with the Xiphosura rather than with the Crustacea. But the presence of antennae, and of five pairs of cephalic appendages; the biramous thoracic and pygidial appendages, the hypostome, and the character of the larval form, as well as the absence of a genital operculum, separate the Trilobites from the Xiphosura and connect them with the Crustacea.
The position of the Trilobites in the Crustacea is, however, difficult to determine. Already in the Cambrian period, at least five main groups of the Crustacea were clearly differentiated, namely, the Phyllopoda, Ostracoda, Cirripedia, Trilobita, and Leptostraca (Phyllocarida), and probably also the Copepoda, but of the last no remains have been preserved as fossils. Palaeontology, therefore, furnishes no connecting links between any two of these orders.
The Crustacea to which the Trilobites show some resemblance are the families Apodidae and Branchipodidae of the Order Phyllopoda (see pp. 19–36). The Trilobita agree with those families in having a large but variable number of trunk-segments, in the possession of a large labrum (hypostome), and in the occurrence of gnathobases on the thoracic appendages; also the foliation of some of the trunk-appendages is somewhat similar. The points of difference, however, are considerable; thus the cephalic appendages are much more specialised in the Apodidae and Branchipodidae than in the Trilobita; in the latter all, with the exception of the antennae, are distinctly biramous, and whilst the basal joints were masticatory the distal parts appear to have been locomotor organs. The appendages of the trunk also differ considerably; in the Trilobita all are clearly biramous, those of the thorax having a schizopodal form. In the possession of a single pair of antennae the Trilobita differ from other Crustacea; but in 242some forms of Apus the second pair of antennae may be rudimentary or even absent.
There are still other features which characterise the Trilobita: thus the eyes are borne on free cheeks, and differ in structure from those of Phyllopods. The broad pygidium formed of fused segments and without terminal fulcra is quite unlike the slender-jointed abdomen of Apus and Branchipus; and whilst in the Trilobites all the segments bear appendages, in the Phyllopods some, at any rate, of the posterior segments are devoid of appendages. The distinct division of the body into an axial and pleural region is not seen in Phyllopods, and is probably a character of some importance, since it occurs in the great majority of Trilobites, including all the early forms.
The existence of some relationship between the Trilobita and the Leptostraca (Phyllocarida) has been maintained by Professor G. H. Carpenter.[195] He points out that some of the earliest Trilobites, such as Holmia kjerulfi (Fig. 148), possess nearly the same number of segments as Nebalia (Fig. 76, p. 111), and that in the latter genus the cephalic appendages, especially the mandibles and maxillae, are less specialised than in Apus, and consequently differ less from those of Trilobites than do the appendages of the Apodidae. Further, in another genus of the Leptostraca, Paranebalia, the biramous thoracic legs, in which both endopodite and exopodite are elongate, approach those of Trilobites more nearly than do the thoracic legs of Apus.
The view[196] that some connexion may exist between the Isopoda and the Trilobita seems to have been based on the similar dorso-ventral flattening of the body, its division into three regions—head, thorax, and abdomen—and the presence of sessile eyes. Beyond this it is difficult to find any resemblance; whilst the differences, such as the variable number of thoracic segments and their biramous appendages in Trilobites, are important.
At present, then, we can only conclude that the Trilobita are more primitive than any other Crustacea, and that their resemblance to some of the Phyllopoda is sufficient to make 243it probable that they had some ancestral connexion;[197] the possibility of such a relationship receives some support from the presence in the Lower Cambrian rocks of Protocaris, a genus of the Phyllopoda which resembles Apus.[198] The primitive characters of Trilobites are the variable and often large number of segments in the thorax and pygidium; the presence of a pair of appendages on every segment except the anal; the biramous form of all except the first pair of appendages; and the lack of specialisation shown by the appendages, especially those of the head.
The classification of Trilobites is due largely to the work of Barrande and Salter, and the families defined by those authors have been, in the main, generally adopted. But the phylogenetic relationship of the families has still, to a large extent, to be established. Salter[199] arranged the families in four groups, but did not claim that that classification was entirely natural. His groups with the families included in each are:—
1. Agnostini. Without eyes or facial suture. Agnostidae.
2. Ampycini. Facial sutures obscure, or submarginal, or absent. Eyes often absent. Trinucleidae.
3. Asaphini. Facial sutures ending on the posterior margin. Acidaspidae, Lichadidae, Harpedidae, Calymenidae, Paradoxidae, Conocephalidae, Olenidae, Asaphidae, Bronteidae, and Proëtidae.
4. Phacopini. Facial sutures ending on the lateral margins. Eyes well developed. Phacopidae, Cheiruridae, and Encrinuridae.
A modification of Salter’s classification has been brought forward by Beecher[200] who divides the Trilobita into three main groups:—
1. Hypoparia. Facial sutures at or near the margin, or ventral. Compound eyes absent. This is equivalent to Salter’s Agnostini and Ampycini with the addition of the Harpedidae.
2442. Opisthoparia. Facial sutures extending from the posterior margin to the front margin, but occasionally uniting in front of the glabella. Eyes holochroal or prismatic, but sometimes absent. This comprises the same families as Salter’s Asaphini with the exclusion of the Harpedidae and Calymenidae.
3. Proparia. Facial sutures extending from the lateral margins, and either cutting the anterior margin or uniting in front of the glabella. Eyes holochroal or schizochroal; occasionally absent. This is equivalent to Salter’s Phacopini with the addition of the Calymenidae.
In each of the groups proposed Beecher regards as the more primitive forms those which possess characters similar to those of the early larval stages, such as narrow free cheeks, the absence of compound eyes, and a glabella which is broad in front and reaches the anterior margin of the head.
The modifications introduced by Beecher can scarcely be regarded as making Salter’s classification more natural. For instance, the Agnostidae differ so much from all other families that, at present, there is no evidence to show that they have any close phylogenetic relationship with the Trinucleidae and Harpedidae. Further, the Calymenidae, which Salter recognised as related to the Olenidae, have been shown by the careful work of Professor Pompeckj[201] to have descended from the latter family, and to have no genetic connexion with the Phacopidae with which they are grouped by Beecher. Then in the Trinucleidae the earliest genus, Orometopus[202] (Fig. 140, A), possesses compound eyes and facial sutures which begin at the posterior margin and unite in front of the glabella; so that, according to Beecher’s classification, that genus would belong to the Opisthoparia, whereas the later genera (Trinucleus, etc.) of the same family would be placed in the Hypoparia. At present, therefore, the only classification of Trilobites which can be adopted is a division into families, of which a short account is given below.

Fig. 146.—Agnostus integer, Beyr., × 8. Cambrian. (After Barrande.)
Fam. 1. Agnostidae (Fig. 146).—Small Trilobites, in which the head and pygidium are of nearly the same size and shape. The thorax is shorter than the head or pygidium, and consists of from two to four segments with grooved pleurae. The length and width of the head are commonly nearly equal, but sometimes 245the length is greater. Eyes are absent. Facial sutures appear to be absent, but are stated by Beecher to be at the margin of the cephalic shield. From the absence of eyes, the probable absence of facial sutures, the few or indistinct furrows on the glabella, and the smaller number of thoracic segments, the Agnostidae appear to be degenerate forms. Microdiscus is apparently less modified than Agnostus, on account of the larger number of thoracic segments, the more distinct segmentation of the pygidium, and, in some species, the larger number of furrows on the glabella. Cambrian and Ordovician. Genera: Agnostus, Microdiscus.
Fam. 2. Shumardiidae.—The body is very small and oval. The cephalic shield is nearly semicircular and very convex, with a broad glabella which expands in front, and in which the furrows, except the neck-furrow, are indistinct. The facial suture is marginal and eyes are absent. There are six thoracic segments with ridged pleurae; the axis is broader than the pleurae. The pygidium is large, and is formed of about four segments similar to those of the thorax. Upper Cambrian and Ordovician. Genus: Shumardia.
Fam. 3. Trinucleidae (Fig. 140).—The head is large and has a flat border (except in Ampyx), and long genal spines. In the earliest genus (Orometopus) the facial sutures start from the posterior margin (near the genal angle) and pass obliquely inwards to the compound eye, from whence they continue forward and unite in front of the glabella. In Ampyx the suture starts from just within the genal angle and passes to the front border, cutting off a narrow free cheek; eyes are absent. In most specimens of Trinucleus no sutures are seen, but some examples show indications of what may be a facial suture (see p. 226), and a suture is sometimes found at the margin of the cephalic border; eyes may occur (see p. 230). The thorax consists of from five to eight segments, with grooved pleurae. The pygidium is triangular. Principally Ordovician. Genera: Orometopus (Upper Cambrian), Ampyx, Trinucleus, Dionide.
Fam. 4. Harpedidae (Figs. 139, G, H; 150, A).—The head is large and has a broad, flat border which is finely punctate, and extends backwards on each side in the form of a horn-like 246projection nearly as far as the posterior end of the thorax. The glabella is convex and does not reach the front margin. The cheeks are less convex than the glabella, and bear eyes which usually consist of two or three lenses. An eye-line connects the eye with the anterior part of the glabella. A suture is stated to occur at the external margin of the flat border. The thorax consists of from twenty-five to twenty-nine segments; its axis is narrow, and the pleurae are long and grooved. The pygidium is very small, and consists of three or four segments. Ordovician to Devonian. Genus: Harpes.

Fig. 147.—Paradoxides bohemicus, Barr. × ½. Middle Cambrian. (After Zittel.)

Fig. 148.—Holmia kjerulfi, Linnars. × 1. Lower Cambrian. (After Holm.)
Fam. 5. Paradoxidae (Figs. 147, 148, 149).—The cephalic shield is large, and bears long genal spines. The glabella is more or less swollen in front. The facial sutures appear to be absent in some genera, and when present extend from the posterior to the anterior margin. The palpebral lobes are long, and often more or less semicircular or kidney-shaped. The thorax is long, and consists of from sixteen to twenty segments with their pleurae produced into spines. The pygidium is very small, and plate-like, 247or sometimes in the form of a long spine. Cambrian. Genera: Olenellus, Holmia, Mesonacis, Olenelloides, Paradoxides, Zacanthoides, Centropleura (Anopolenus). Remopleurides (Fig. 150, D) from the Ordovician is usually included in the Paradoxidae, but probably belongs to a separate family.

Fig. 149.—Clenelloides armatus, Peach. Lower Cambrian, × 3. (After Peach.)
Fam. 6. Conocephalidae (Conocoryphidae) (Fig. 150, E).—The cephalic shield is semicircular, and larger than the pygidium. The glabella narrows in front. The facial suture passes from near the genal angle on the posterior border to the antero-lateral margin, and limits a large fixed cheek and a narrow free cheek. Eyes are absent or rudimentary, but an eye-line is usually present. The thorax consists of from fourteen to seventeen segments with grooved pleurae, which may be pointed, but are not usually produced into spines. The pygidium is small, and formed of few segments. Cambrian. Genera: Conocoryphe, Atops, Ctenocephalus, Bathynotus.
Fam. 7. Olenidae (Figs. 142, 143; 150, B, C).—The cephalic shield is larger than the pygidium. The glabella is either rectangular or parabolic. The facial suture passes from the posterior to the anterior margin. The palpebral lobes are of moderate or rather large size, and are connected by an eye-line with the front part of the glabella. The thorax includes from eleven (occasionally fewer) to eighteen segments with grooved pleurae. The pygidium is usually small, with from two to eight segments. Principally Cambrian. Genera: Ptychoparia, Angelina, Solenopleura, Sao, Agraulos (Arionellus), Ellipsocephalus, Protolenus, Olenus, Peltura, Acerocare, Eurycare, Ctenopyge, Leptoplastus, Triarthrus, Parabolina, Sphaerophthalmus, Parabolinella, Ceratopyge (position doubtful). Dikelocephalus is usually placed in the Olenidae, but perhaps belongs to a distinct family.
Fam. 8. Calymenidae (Figs. 136, 137).—The glabella is broadest behind. The facial suture starts at or near the genal angle—sometimes on the posterior border just inside the angle, sometimes on the lateral border just in front of the angle; the suture may be continuous with the other suture in front of the glabella, or may cut the anterior margin, beneath which it is 248connected with the other suture by means of a transverse suture (Fig. 137, B, D). The eyes are rather small. The thorax consists of thirteen segments with grooved pleurae; the pygidium of from six to fourteen segments. Ordovician to Devonian. Genera: Calymene, Synhomalonotus, Homalonotus.

Fig. 150.—A, Harpes ungula, Sternb., Ordovician. B, Ellipsocephalus hoffi, Scloth., Cambrian. C, Olenus truncatus, Brünn., Cambrian. (After Angelin.) D, Remopleurides radians, Barr., Ordovician. E, Conocoryphe sulzeri, Barr., Cambrian. F, Illaenus dalmanni, Volb., Ordovician. G, Proëtus bohemicus, Corda, Silurian, × 1½. H, Aeglina prisca, Barr., Ordovician, × 3. I, Phacops sternbergi, Barr., Devonian. (A, D, E, G, H, I, after Barrande; B, F, from Zittel; natural size except G, H.)
249Fam. 9. Asaphidae (Fig. 150, F).—The body is oval and commonly rather large. The cephalic shield is large, with its glabella often indistinctly limited and the glabella-furrows often obscure. The facial suture starts from the posterior margin and usually cuts the anterior margin, but is sometimes continued in front of the glabella. The relative size of the fixed and free cheeks varies greatly. The eyes are of variable size. The thorax consists of eight or ten (sometimes fewer) segments; the pleurae are generally grooved, but sometimes plane. The pygidium is large, often being similar in form and size to the head; it consists of numerous segments which, however, may be indistinctly shown; the axis in some forms is obsolete. Upper Cambrian (Tremadoc) to Silurian; common in the Ordovician. Genera: Asaphus (sub-genera, Megalaspis, Asaphellus, Symphysurus, etc.), Ogygia, Barrandia, Niobe, Nileus, Illaenus, Bumastus, Stygina. Aeglina (Fig. 150, H) is usually placed in this family, but its systematic position is doubtful.
Fam. 10. Bronteidae.—The general form is similar to that of the Asaphidae. The glabella broadens rapidly in front, and is marked with furrows on each side, which are usually short, and may be indistinct. The facial suture passes from the posterior margin to the crescentic eye which is situated rather near the posterior border, and from thence to the anterior margin. There are ten thoracic segments with ridged pleurae. The pygidium is longer than the head, and has a very short axis, from which the furrows on the pleural part radiate. Ordovician to Devonian. Genus: Bronteus.
Fam. 11. Phacopidae (Figs. 138; 150, I; 151, C).—The head and pygidium are of about the same size. The glabella is distinctly limited, and wider in front than behind, with a neck-furrow and three other furrows, of which some of the anterior may be indistinct or obsolete. The eyes are schizochroal and usually large. The facial suture begins at the lateral margin and unites with the suture of the other side in front of the glabella. There are eleven thoracic segments with grooved pleurae. The pygidium is usually large, with a distinct axis and many segments. Ordovician to Devonian. Genera: Phacops, Trimerocephalus, Acaste, Pterygometopus, Chasmops, Dalmanites, Cryphaeus.

Fig. 151.—A, Phillipsia gemmulifera, Phill., Carboniferous. B, Arethusina konincki, Barr., Ordovician. C, Dalmanites limulurus, Green, Silurian. (After Hall.) D, Cheirurus insignis, Beyr., Silurian. E, Deiphon forbesi, Barr., Silurian. F, Acidaspis dufrenoyi, Barr., Silurian. (A, B, from Zittel; D, E, F, after Barrande; natural size.)
Fam. 12. Cheiruridae (Fig. 151, D, E).—The glabella is convex or inflated, and distinctly defined. The facial suture passes from the lateral to the front margin. The free cheeks 251are small, and the eyes usually rather small. There are from nine to eighteen (usually eleven) thoracic segments; the pleurae have ridges or grooves and free ends. The pygidium is small, consisting of from three to five segments often produced into spines. Upper Cambrian to Devonian. Genera: Cheirurus, Deiphon, Placoparia, Sphaerexochus, Amphion, Staurocephalus.
Fam. 13. Proëtidae (Figs. 150, G; 151, A, B).—The body is rather small, and the head forms about a third of its entire length. The glabella is sharply defined, and its furrows are sometimes indistinct; the posterior furrow curves backward to the neck-furrow, thus limiting a basal lobe on each side of the glabella. The eyes are often large (Fig. 150, G); but in Arethusina (Fig. 151, B), in which an eye-line is present, they are small. The facial sutures pass from the posterior to the anterior margin. The free cheeks are large. There are from eight to twenty-two thoracic segments with grooved pleurae. The pygidium is usually formed of numerous segments, and its margin is usually entire. Ordovician to Permian. Genera: Proëtus, Arethusina, Cyphaspis, Phillipsia, Griffithides, Brachymetopus, Dechenella.[203]
Fam. 14. Encrinuridae.—The cephalic shield is ornamented with tubercles. The free cheeks are narrow, and the eyes very small. The facial suture extends from the lateral margin (or from the genal angle) to the anterior margin. There are from ten to twelve thoracic segments with ridged pleurae. On the axis of the pygidium numerous segments are seen, but usually fewer are indicated on the lateral parts. Ordovician and Silurian. Genera: Encrinurus, Cybele, Dindymene.
Fam. 15. Acidaspidae (Fig. 151, F).—The cephalic shield is broad, with a spinose margin, genal spines, and sometimes spines on the neck-ring. The glabella has a longitudinal furrow on each side, due to the backward bending of the lateral furrows. The facial suture passes from the posterior border (near the genal angle) to the anterior border. The free cheeks are large; the eyes small. There are from eight to ten thoracic segments with ridged pleurae, which are produced into long backwardly directed spines. The pygidium is short, and is formed of two or three segments with long spines at the margin. Ordovician to Devonian. Genus: Acidaspis.
252Fam. 16. Lichadidae.—The body is broad, with a granular dorsal surface. The cephalic shield is small and short, with spinose genal angles. The glabella is broad, and its anterior furrows are directed backwards, limiting a convex median lobe and some lateral lobes. The facial suture extends from the posterior to the anterior margin. There are nine or ten thoracic segments with grooved pleurae, which have pointed ends. The pygidium is large and triangular, with a short axis and a toothed margin. Ordovician to Devonian. Genus: Lichas (sub-genera, Arges, Dicranogmus, Conolichas, Ceratolichas).
INTRODUCTION TO ARACHNIDA,
AND
XIPHOSURA
CHAPTER IX
ARACHNIDA—INTRODUCTION
The Arachnida, together with the Crustacea, Insecta, Myriapoda, and Peripatus, make up the great phylum Arthropoda, a phylum which, from the point of view of numbers of species and of individuals, is the dominant one on this planet, and from the point of view of intelligence and power of co-operating in the formation of social communities is surpassed but by the Vertebrata. The Arachnida form a more diverse class than the Insecta; they differ, perhaps, as much inter se as do the Crustacea, and in structure as in size and habit they cover a wide range.
Lankester in his article upon the Arthropoda, in the tenth edition of the Encyclopaedia Britannica, dwells upon the fact that whereas the adult Peripatus has but one persisting segment in front of the head, and its mouth is between the second persisting appendages, in Arachnids the mouth has receded and lies between the bases of the appendages (pedipalpi) of the third persisting segment, while two of the persisting segments, those of the eyes and chelicerae, have passed in front of the mouth. This process has continued in the Crustacea and in the Insecta; in both of these classes there are three embryonic segments in front of the adult mouth, which lies between the appendages of the fourth segment.
In the larger and more complex Arachnida the number of segments is fixed and constant, and though possibly no adult member of the group, owing to the suppression of one or more segments during the ontogeny, ever shows the full number at any one time, the body can be analysed into twenty-one segments. It is interesting to note that the same number of segments occurs in 256Insecta and in the higher Crustacea.[204] The significance of this fact is not perhaps apparent, but it seems to indicate “a sort of general oneness, if I may be allowed to use so strong an expression,” as Mr. Curdle said when discussing the unities of the drama with Nicholas Nickleby.
These segments are arranged in higher categories or “tagmata,” of which we can recognise three: (i.) the prosoma, (ii.) the mesosoma, and (iii.) the metasoma. The prosoma, sometimes termed the “cephalothorax,” includes all the segments in front of the genital pore. According to this definition the prosoma includes the segment which bears the chilaria in Limulus (the King-crab) and the pregenital but evanescent segment in Scorpions. The mesosoma begins with the segment bearing the genital pore, and ends with the last segment which bears free appendages, six segments in all. The metasoma also consists of six segments which have no appendages; together with the mesosoma it forms the abdomen of some writers. The anus lies posteriorly on the last segment, and behind it comes in the higher forms a post-anal “telson,” taking in Scorpions the form of the sting, in King-crabs that of the spine.
As we have seen, it is only in the more typical and perhaps higher forms that we can find our twenty-one segments, and then they are never present all at once. In many groups of Arachnids the number is reduced at the hinder end, and obscured by the fusion of neighbouring segments. Also segments are dropped as a stitch is dropped when knitting; for instance, in the rostral segment which has a neuromere, and in the Spider Trochosa vestigial antennae, or in Scorpions the pregenital segment.
Primitive Arachnids appear to have lived in the sea and to have breathed by gill-books borne on appendages; when their descendants took to living on land and to breathing air instead of water, the gill-books sank into the body and became lung-books, to which the air was admitted by slit-like stigmata. In other terrestrial forms the lung-books are replaced by tracheae which in their structure and arrangement resemble those of Peripatus rather than those of the Insecta. The circulation, as 257is usual in Arthropods, is largely lacunar, but in Scorpions and Limulus the arteries form definite channels, and are in fact better developed than in any other Arthropod.
As a rule the alimentary canal in Arachnids is no longer than the distance between the mouth and the anus; but in the King-crab, where the mouth is pushed back almost to the centre of the body, there is a flexure in the median vertical plane. Paired glands, usually called the liver, open into the mesenteron; food passes into the lumen of these glands, and is probably digested there. In many Arachnids these glands extend into the limbs. In those members of the group that have become terrestrial the nitrogenous excreta are separated out by Malpighian tubules which open into the proctodaeum; but coxal glands, homologous with the green gland and shell-glands of Crustacea, may coexist, and in the aquatic Limulus these alone are found. They usually open on the base of one or more pairs of walking legs.
The endosternite, or internal skeletal plate to which muscles are attached, reaches a higher development in the Arachnida than in the Crustacea. In Scorpions it forms a kind of diaphragm incompletely separating the cavities of the pro- and meso-soma.
The supra-oesophageal ganglion supplies the two existing segments which have slipped before the mouth, i.e. those of the eyes and of the chelicerae. The post-oral ganglia in the Acarina, the Pedipalpi, the Solifugae, and the Araneae have fused into a central nerve-mass, from which nerves radiate; but in Limulus the prosomatic appendages are all supplied from the nerve-ring. The chief sense-organs are eyes of the characteristic Arthropod type, and sensory hairs of a great variety of complexity. Scorpions and Spiders have stridulating organs, and we may assume that they have also some auditory apparatus; perhaps some of the hairs just mentioned act as hearing organs.
Arachnids are male and female; they do not reproduce asexually, and there is no satisfactory proof that they ever reproduce parthenogenetically. As a rule there is little external difference between the two sexes, except in Spiders, where the male is as a rule smaller than the female, and when adult has the pedipalpi modified for use in depositing the spermatophores. The ovaries and testes are annular, and with their ducts encircle the alimentary canal in Mites and Phalangids; in Scorpions and 258King-crabs they have become retiform. Mites, Scorpions, and Pedipalps are viviparous, their eggs developing in the ovary or in a uterus. Other Arachnids lay eggs, and many Spiders and Pseudoscorpions carry their eggs about with them. As a rule the young is but a miniature of the parent, and the Arachnid, unlike the Crustacean or Insect, undergoes little or no metamorphosis.
A certain number of Mites are parasitic in plants and in animals, and a few, together with a few Spiders, have resumed the aquatic life of their remote ancestors. The members of some Orders, such as the Solifugae and Opiliones, are nocturnal, and many are provided with silk-glands and weave webs which reach their highest pitch of perfection amongst the Spiders. At times—especially is this the case with the Mites—enormous numbers of individuals live together, but they never show the least adaptation to communal life, and no individuals are ever specialised to perform certain functions, as is the rule in communities of social Insects.
With the two exceptions that we regard the Trilobites as more nearly allied to the Crustacea, and have therefore considered them apart, and have treated the Pycnogonida independently of the Arachnida, we have followed Lankester in his classification, though not always in his nomenclature:—
CHAPTER X
ARACHNIDA (CONTINUED)—DELOBRANCHIATA = MEROSTOMATA—XIPHOSURA
SUB-CLASS I.—DELOBRANCHIATA = MEROSTOMATA.
Order I. Xiphosura.[206]
In his recent classification of the Arachnida, Lankester[207] has grouped the Xiphosura or King-crabs with the extinct Eurypterids or Gigantostraca under the name of Delobranchiata, better known under the name Merostomata[208] of Dana. The chief character of this group, and one which differentiates it from all the animals placed together by Lankester in the group Embolobranchiata, is that they have gills patent and exposed. The Xiphosura are, in fact, with the exception of a few marine Mites, the only Arachnids which now live in the sea as did their allies the Eurypterids in Palaeozoic times. With a few fresh-water exceptions, all other Arachnids have taken to life on land, and with a change from water-breathing to air-breathing came a change in the respiratory system, the gills becoming “lung-books,” or possibly tracheae, or disappearing altogether.
260A few years ago Pocock re-classified the Xiphosura, and his classification will be found on pp. 276, 277. It will be noticed that in his classification the generic name Limulus has disappeared. I have, however, retained it in this article, partly because I regard the name as so well established that every one knows what it denotes, and partly because in a group which contains confessedly very few species, differing inter se comparatively slightly, it seems unnecessary to complicate matters with sub-families and new names.
Looked at from above a Limulus presents a horse-shoe-like outline, from the posterior end of which projects a long spine. It is often called in America the Horsefoot-crab, but its common or vulgar name is the King-crab. Across the middle of the body is a joint, and this joint separates the prosoma from the meso- and metasoma which are in King-crabs fused together. The prosoma comprises all the segments up to and including the segment which carries the chilaria;[209] the mesosoma begins with the segment bearing the genital pores, and ends with the last segment which bears appendages; the metasoma comprises all the segments posterior to the last segment which carries appendages. The prosoma corresponds with the “cephalothorax” of some authors, and the meso-plus the metasoma are equivalent to their “abdomen.”
Dorsally, then, the prosoma is a vaulted structure with a smooth, horse-shoe-shaped anterior and lateral margin. Its posterior edge, the line where the meso-plus the metasoma are hinged, is a re-entrant bay with three sides. The meso- and metasoma are in the King-crabs fused together and form a hexagon. Three sides of this hexagonal double region form the hinge, two form the lateral margins and slope inwards; these bear six fused and six-jointed spines which have a segmental value. The sixth or posterior side is indented, and its concavity forms the area to which the large post-anal, unsegmented tail or spine is hinged.
The whole body is covered by a smooth chitinous sheath varying from sage-green to black in colour, and it is kept very clean, probably by some excretion which hinders various sessile animals attaching themselves to it as they do, for instance, on 261many Copepods. Burrowing animals like Limulus are usually free from these messmates. King-crabs have a self-respecting, well-groomed appearance. On the rounded dorsal surface the chitinous covering is produced into a certain number of spines arranged in a median and two lateral rows. The anterior median spine overhangs the median eyes, and the anterior lateral spine on each side overshadows the large lateral eyes.

Fig. 152.—Dorsal view of the King-crab, Limulus polyphemus, × ½. From Shipley and MacBride. 1, Carapace covering prosoma; 2, meso- and metasoma; 3, telson; 4, median eye; 5, lateral eye.
The vaulted carapace is turned in on the under side, where there is a flat rim which widens anteriorly, and on the inner edge this rim borders a sunken area, into the concavity of which 262the numerous appendages project. Thus, although when viewed from above a Limulus looks as though it had a solid body shaped something like half a pear, when viewed from below, especially if the appendages be removed, it will be found that the body is thin and hollowed, and almost leaf-like, as if most of the edible part of the half-pear had been scooped out. Within the hollow thus formed the appendages lie, and here they move about, seldom or never protruding beyond the edge of the carapace,—in fact, all except the pedipalps and ambulatory legs are too short to project beyond this limit.

Fig. 153.—Ventral view of the King-crab, Limulus polyphemus, × ½. From Shipley and MacBride. 1, Carapace covering prosoma; 2, meso- and metasoma; 3, telson; 4, chelicera; 5, pedipalp; 6, 7, 8, 9, 3rd to 6th appendages, ambulatory limbs; 10, genital operculum turned forward to show the genital apertures; 11, 12, 13, 14, 15, appendages bearing gill-books; 16, anus; 17, mouth; 18, chilaria.
263The body of a King-crab can be analysed into twenty-one segments, but these do not all persist to the adult stage. They are grouped together in higher aggregates, or “tagmata” as Lankester calls them, and most of the segments bear paired appendages.
The segments with their respective appendages and their grouping into tagmata are shown in the following scheme:—
| Appendages. | ||||
| I. | Segment | Median eyes | Preoral | Prosoma |
| II. | „ | Rostrum | „ | |
| III. | „ | Chelicerae | „ | |
| IV. | „ | Pedipalpi | Lateral to mouth | |
| V. | „ | 1st Walking Legs | Postoral | |
| VI. | „ | 2nd Walking Legs | „ | |
| VII. | „ | 3rd Walking Legs | „ | |
| VIII. | „ | 4th Walking Legs | „ | |
| IX. | „ | Chilaria | „ | |
| X. | „ | Genital operculum | „ | Mesosoma |
| XI. | „ | 1st Gill-books | „ | |
| XII. | „ | 2nd Gill-books | „ | |
| XIII. | „ | 3rd Gill-books | „ | |
| XIV. | „ | 4th Gill-books | „ | |
| XV. | „ | 5th Gill-books | „ | |
| XVI. | „ | No appendages | „ | Metasoma |
| XVII. | „ | „ | „ | |
| XVIII. | „ | „ | „ | |
| XIX. | „ | „ | „ | |
| XX. | „ | „ | „ | |
| XXI. | „ | „ | „ | |
We have followed Carpenter[210] in inserting the rostral segment. This corresponds with the segment that in Insects and Crustacea bears the antennae or first antennae respectively, the absence of these organs being one of the characteristic but negative features of all Arachnids. The evidence for the existence of this evanescent segment rests partly upon the observation of von Jaworowski[211] on the vestigial feelers in an embryo Spider, Trochosa, and perhaps more securely on the fact that, according to Korschelt and Heider, there is a distinct neuromere for this segment, between the proto-cerebral neuromere which supplies the eyes and the trito-cerebral neuromere which supplies the chelicerae. According to Brauer[212] the chelicerae of Scorpions are also supplied by the third neuromere.
The bases of the chelicerae do not limit the mouth, but between and behind them is a ridge or tubercle which has the 264same relationship to the mouth of Limulus that the labrum has in Insects and some Crustacea. Posteriorly the mouth is bounded by the “promesosternite,” a large median plate which lies between the bases of the ambulatory limbs. The pedipalps and all the ambulatory limbs have their bases directed towards the mouth, their gnathobases or sterno-coxal processes are cushion-like structures covered with spines—all pointing inwards—and with crushing teeth. They form a very efficient manducatory apparatus. The boundary of the mouth is finally completed by the chilaria.
Certain of the appendages which persist will be described with the functions they subserve, the eyes with the sense-organs, the genital operculum with the generative organs, the gill-books with the respiratory system, but the chelicerae, pedipalpi, and walking limbs, which have retained the functions of prehension and locomotion usual to limbs, merit a little attention.[213] The chelicerae are short and composed of but three joints. They are, like the succeeding segments, chelate, and the chelae of all are fine and delicate like a pair of forceps rather than like a Lobster’s claw. In the female L. polyphemus the pedipalp is remarkably like the three ambulatory legs which succeed it, and all four are chelate, but in the adult male the penultimate joint of the pedipalp is not prolonged to form one limb of the chela, which is therefore absent, and the appendage is thicker and heavier than in the other sex. In L. longispina and L. moluccanus the first walking leg, as well as the pedipalp, ends in a claw and not in a chela; the immature males resemble the females. The first three walking legs in both sexes of L. polyphemus resemble the pedipalpi of the female, and like them have six joints. The fourth and last pair of ambulatory appendages is not chelate, but its distal joints carry a number of somewhat flattened structures, which are capable of being alternately divaricated and approximated or bunched together. This enables them to act as organs for clearing away sand or mud from beneath the carapace as the creature lies prone on the bottom of the sea. To quote Mr. Lloyd,[214] the “two limbs are, sometimes alternately and sometimes simultaneously, thrust backward below the carapace, quite beyond the hinder edge 265of the shell; and in the act of thrusting, the lobes or plates on each leg encounter the sand, the resistance or pressure of which causes them to open and fill with sand, a load of which at every thrusting operation is pushed away from under the king-crab, and deposited outside the carapace. The four plates then close and are withdrawn closed, previous to being opened and charged with another load of sand; and at the deposit of every load the whole animal sinks deeper into its bed, till it is hidden all except the eyes.” There seems little doubt that the action of these appendages in removing the sand from under the carapace is reinforced by the fanning action of the respiratory appendages, which set up a current that helps to wash the particles away. But the posterior walking legs are not the only organs used in burrowing. The Rev. Dr. Lockwood,[215] who observed the habits of L. polyphemus off the New Jersey coast, says, “The king-crab delights in moderately deep water, say from two to six fathoms. It is emphatically a burrowing animal, living literally in the mud, into which it scoops or gouges its way with great facility. In the burrowing operation the forward edge of the anterior shield is pressed downward and shoved forward, the two shields being inflected, and the sharp point of the tail presenting the fulcrum as it pierces the mud, whilst underneath the feet are incessantly active scratching up and pushing out the earth on both sides. There is a singular economy of force in this excavating action; for the doubling up or inflecting and straightening out of the two carapaces, with the pushing purchase exerted by the tail, accomplish both digging and subterranean progression.”
At night-time Limulus is apt to leave the sand and progress by a series of short swimming hops, the respiratory appendages giving the necessary impetus, whilst between each two short flights the animal balances itself for a moment on the tip of its tail. During this method of progressing the carapace is slanting, forming an angle of about 45° with the ground. The unsegmented tail is also used when a King-crab falls on its back. “The spine is then bent, i.e. its point is planted in the sand so that it makes an acute angle with the carapace, which is then so far raised that some of the feet are enabled to grasp a projecting surface, either longitudinal or vertical, or at some combination of the two; and the crab then turns over.”

Fig. 154.—A sagittal section of Limulus, seen from the right side, somewhat smaller than natural size. After Patten and Redenbaugh.
All the prosomatic appendages, except the chelicera (4) and chilarium (33) of the right side, are omitted. The genital operculum (32) and the five gills (28) are represented.
The muscles are omitted except the fibres running from the occipital ring to the posterior side of the oesophagus, the chilarial muscles, the sphincter ani (27), and the levator ani (24).
The endosternite (34), with the occipital ring and the capsuliginous bar, is seen from the side, and the positions of the abdominal endochondrites (31) are indicated.
The mouth (1) leads into the oesophagus, which passes through the brain to the proventriculus (12). A constriction, which marks the position of the pyloric valve, separates the proventriculus from the intestine (23) which passes posteriorly to the anus (26). A pair of hepatic ducts (15) enter the intestine opposite the endocranium.
The heart (16) surrounded by the pericardial sinus lies above the intestine. The pericardium is shown between the heart and the intestine. The ostia (17) of the heart and the origins of the four lateral arteries (19) are indicated; the frontal artery (13) and the aortic arches (14) curving down to the brain, arise from the anterior end of the heart; the superior abdominal artery and the opening of the collateral artery into it are shown.
The brain surrounding the oesophagus is seen in side view upon the neural side of the endosternite (34). The ventral cord (35) passes through the occipital ring into the abdominal region. The anterior commissure (3), with the three rostral nerves (2) innervating the rostrum, or labrum, and four of the post-oral commissures, are represented.
The cheliceral nerve with the small external pedal branch is shown entire, but the next five neural nerves are cut off. The chilarial nerve, the opercular nerve, and the five branchial nerves, enter their respective appendages, the two former passing through the occipital ring.
From the fore-brain the three olfactory nerves (5) pass anteriorly to the olfactory organ; the median eye-nerve (10) passes to the right of the proventriculus (12) to the median eyes (11); the lateral eye-nerve (7) passes forward and is represented as cut off opposite the proventriculus. The lateral nerve (9) or first haemal nerve is also cut267 off just beyond the point where it fuses with the second haemal nerve (8). The stomodaeal nerve (6) ramifies over the oesophagus and proventriculus.
The second haemal nerve (8) passes to the anterior extremity of the carapace; its haemal branch is cut off opposite the proventriculus. An intestinal branch arises from near its base and disappears behind the anterior cornu of the endosternite.
The next three haemal nerves (36) are cut off close to the brain, and the following nine haemal nerves are cut off beyond the cardiac branches. The fifteenth haemal nerve (29) is cut off beyond its branch to the telson muscles. Both branches of the haemal nerve are represented extending into the telson (25).
The intestinal nerves are shown arising from the haemal nerves and entering the intestine. Those from the sixth and seventh neuromeres pass through foramina in the endosternite, and communicate with a plexus in the longitudinal abdominal muscles before entering the intestine. The eighth passes just posterior to the endosternite and joins the same plexus. Those from the first four branchial neuromeres arise very near the abdominal ganglia, and are double in their origins, the anterior branches joining the above-mentioned plexus, and the posterior branches entering the intestine. The fifteenth extends far back towards the rectum and anastomoses with the sixteenth, which arises from the caudal branch of the sixteenth haemal nerve, and innervates the rectum and anal muscles.
The segmental cardiac nerves (18) arise from the haemal nerves of the sixth to the thirteenth neuromeres respectively. The most anterior one passes to the inter-tergal muscles and the epidermis in the median line, but the connections with the cardiac plexus have not been made out. The next two (18) fuse to form a large nerve, which passes to the inter-tergal muscles and epidermis, but has not been observed to connect directly with the cardiac plexus. It, however, sends posteriorly a branch, the pericardial nerve (20), which in turn gives a branch to each of the cardiac nerves of the branchial neuromeres, and then continues onward to the posterior margin of the abdomen. This nerve lies in the epidermis. The median and lateral cardiac nerves (22 and 21) are seen upon the walls of the heart. The five cardiac nerves from the branchial neuromeres pass, in the epidermis, to the median line, and dip down to the median nerve (22) of the heart opposite the last five pairs of ostia (17). They communicate with the pericardial nerve (20) and also with the lateral sympathetic nerve (30).
Two post-cardiac nerves pass from the first and second post-branchial nerves to the epidermis posterior to the heart.
The last cardiac nerve and the two post-cardiac nerves give off branches which anastomose with each other and innervate the extensors of the telson.
The lateral sympathetic nerve (30) receives branches from all the neuromeres from the eighth to the fourteenth, either through the cardiac nerves or the haemal nerves, and innervates the branchio-thoracic muscles, extending with these far into the cephalothorax.
1, Mouth; 2, rostral nerve in labrum; 3, anterior commissure; 4, chelicera; 5, olfactory nerves; 6, stomodaeal nerve; 7, lateral eye-nerve; 8, 2nd haemal nerve; 9, lateral nerve; 10, median eye-nerve; 11, median eye; 12, proventriculus; 13, frontal artery; 14, aortic arch; 15, anterior hepatic duct of liver; 16, heart; 17, 2nd ostium; 18, 7th and 8th segmental cardiac nerves; 19, one of the lateral arteries; 20, pericardial nerve; 21, lateral cardiac nerve; 22, median cardiac nerve; 23, intestine; 24, levator ani muscle; 25, telson; 26, anus; 27, sphincter ani muscle; 28, last branchial appendage; 29, 15th haemal nerve; 30, lateral sympathetic nerve; 31, 8th abdominal endochondrite; 32, genital operculum; 33, chilarium; 34, endosternite; 35, ventral nerve-cord; 36, 6th haemal nerve; 37, origin of 6th neural nerve.
Limulus feeds partly on bivalves, but mainly on worms, especially Nereids, which it catches with its chelate limbs as it burrows through the sand. The food is held immediately under the mouth by the chelicerae, aided at times by the succeeding appendages; it is thus brought within range of the gnathobases of the 268walking legs, and these by an alternate motion “card” the food into fragments, which when sufficiently comminuted pass into the mouth. At times its appendages are caught between the valves of Venus mercenaria, a burrowing bivalve known in America as the “quahog” or “round clam.” The Limulus has seized with its chelate claws the protruding siphon of this mollusc, which, being rapidly drawn in, drags with it the limb of the king-crab, and the valves of the clam are swiftly snapped to.
As a rule in Arachnids the alimentary canal is no longer than the body, and runs straight from mouth to anus, but in Limulus, the mouth being pushed far backward, there is a median loop, and the narrow oesophagus which leads from the mouth, having traversed the nerve-ring, passes forward towards the anterior end of the carapace. Here it enters into a somewhat ⸧ shaped and spacious proventriculus; posteriorly the proventriculus opens by a funnel-shaped valve into the anterior end of the narrow intestine. All these structures are derived from the stomodaeum, are lined with chitin and are provided with very muscular walls whose internal surface is thrown into longitudinal ridges. The intestine runs straight backward, diminishing in its diameter, and ends in a short, chitin-lined, and muscular rectum which is derived from the proctodaeum; the anus is a longitudinal slit. A large gland, usually called the liver, consisting of innumerable tubules, pours its secretions into the broader anterior end of the intestine by two ducts upon each side; it extends into the meso- and metasoma, and, together with the reproductive organs, forms a “packing” in which the other organs are embedded. The contents of the alimentary canal are described as “pulpy and scanty,” and probably much of the actual digestion goes on inside the lumen of the above-mentioned gland.
The vascular system of Limulus, like that of the Scorpions, is more completely developed than is usually the case in Arthropods. For the most part the blood runs in definite arteries, and when it passes as it does into venous lacunae these are more definite in position and in their retaining walls than in other members of the phylum.
The heart lies in a pericardial space with which it communicates by eight[216] pairs of ostia. Eight paired bands of connective tissue, the “alary muscles” of authors, sling the heart to the 269pericardial membrane. Posteriorly the pericardial chamber receives five paired veins on each side coming from the gills and returning the purified blood to the heart.
Eleven arteries arise from the heart. These are (i.) a median frontal artery which, passing forward, divides into a right and left marginal artery. These run round the edge of the carapace to its posterior angle, where each receives a branch of the collateral artery mentioned below. (ii.) and (iii.) are the aortic arches (Fig. 154), paired vessels running round and supplying the proventriculus and oesophagus. These unite ventrally in a vascular ring which encloses the nerve-ring, and is continued along the ventral nerve-cord as the ventral artery and along some of the chief nerves. This vascular ring supplies the lateral eyes and all the appendages mentioned on p. 263 up to and including the genital operculum. The ventral artery supplies the respiratory appendages, and gives branches to the rectum, caudal spine, etc. Two of its branches encircle the rectum, and uniting open into the superior abdominal artery. iv.–xi. are paired lateral arteries which leave the heart beneath the anterior four ostia, and soon enter a longitudinal pair of collateral arteries which unite behind in the just mentioned superior abdominal artery; they also give off branches to the muscles and to the intestine, and a stout branch mentioned above which passes into the marginal artery posteriorly. The venous system is lacunar, and the blood is collected from the irregular spaces between the various organs into a pair of longitudinal sinuses, whence it passes into the operculum and the five pairs of gills. A large branchio-cardiac canal returns the blood from each gill to the cavity of the pericardium, and so through the ostia to the heart. Eight veno-pericardiac muscles run from the under surface of the pericardium to be inserted into the upper surface of the longitudinal sinus; they occur opposite the ostia, and play an important part in the mechanism of the circulation. The blood is coloured blue by haemocyanin; amoeboid corpuscles float in the plasma.
The respiratory organs are external gills borne on the posterior face of the exopodite of the lamella-like posterior five mesosomatic limbs. Each gill consists of a series of leaves like the leaves of a book, and some 150–200 in number. Within the substance of each leaf the blood flows, while without the oxygen-carrying water circulates between the leaves. These gillbearing 270appendages can be flapped to and fro, and they seem to be at times held apart by the flabellum, a spatulate process which Patten and Redenbaugh regard as a development of the median sensory knob on the outer side of the coxopodite of the last pair of walking limbs.

Fig. 155.—Diagram of the first gill of Limulus, from the posterior side, showing the distribution of the gill-nerve to the gill-book (about natural size). After Patten and Redenbaugh. 1, Inner lobe of the appendage; 2, outer lobe of appendage; 3, median lobe of appendage; 4, gill-book; 5, neural nerve of the ninth neuromere; 6, internal branchial nerve; 7, gill-nerve; 8, median branchial nerve; 9, external branchial nerve.
Limulus has no trace of Malpighian tubules, structures which seem often to develop only when animals cease to live in water and come to live in air. The Xiphosura have retained as organs of nitrogenous excretion the more primitive nephridia, or coxal glands as they are called, in the Arachnida. They are redbrick in colour, and consist of a longitudinal portion on each side of the body, which gives off a lobe opposite the base of the pedipalps and each of the first three walking legs—in the embryo also of the chelicerae and last walking legs, but these latter disappear during development. A duct leads from the interior of the gland and opens upon the posterior face of the last pair of walking legs but one.
The nervous system has been very fully described by Patten and Redenbaugh, and its complex nature plays a large part in the ingenious speculations of Dr. Gaskell as to the origin of Vertebrates. It consists of a stout ring surrounding the oesophagus and a ventral nerve-cord, composed—if we omit the so-called fore-brain—of sixteen neuromeres. The fore-brain supplies the median and the lateral eyes, and gives off a median nerve which runs to an organ, described as olfactory by Patten, situated in front of the chelicerae on the ventral face of the carapace. Patten distinguishes behind the fore-brain a mid-brain, which 271consists solely of the cheliceral neuromere, a hind-brain which supplies the pedipalps and four pair of walking legs, and an accessory brain which supplies the chilaria and the genital operculum. This is continued backward into a ventral nerve-cord which bears five paired ganglia supplying the five pairs of gills and three pairs of post-branchial ganglia; the latter are ill-defined and closely fused together. As was mentioned above, the whole of the central nervous system is bathed in the blood of the ventral sinus.
The sense-organs consist of the olfactory organ of Patten, the median and lateral eyes, and possibly of certain gustatory hairs upon the gnathobases. The lateral eyes in their histology are not so differentiated as the median eyes, but both fall well within the limits of Arachnid eye-structure, and their minute anatomy has been advanced as one piece of evidence amongst many which tend to demonstrate that Limulus is an Arachnid.
Both ovaries and testes take the form of a tubular network which is almost inextricably entangled with the liver. From each side a duct collects the reproductive cells which are formed from cells lining the walls of the tubes, and discharges them by a pore one on each side of the hinder surface of the genital operculum. As is frequently the case in Arachnids the males are smaller than the females, and after their last ecdysis the pedipalps and first two pairs of walking legs, or some of these appendages, end in slightly bent claws and not in chelae. Off the New Jersey coast the king-crabs (L. polyphemus) spawn during the months of May, June, and July, Lockwood states at the periods of highest tides, but Kingsley[217] was never “able to notice any connexion between the hours when they frequent the shore and the state of the tide.” “When first seen they come from the deeper water, the male, which is almost always the smaller, grasping the hinder half of the carapace of the female with the modified pincer of the second pair of feet. Thus fastened together the male rides to shallow water. The couples will stop at intervals and then move on. Usually a nest of eggs can be found at each of the stopping-places, and as each nest is usually buried from one to two inches beneath the surface of the sand, it appears probable that the female thrusts the genital plate into the sand, while at the same time the male discharges the milt into the 272water. I have not been able to watch the process more closely because the animals lie so close to the sand, and all the appendages are concealed beneath the carapace. If touched during the oviposition, they cease the operation and wander to another spot or separate and return to deep water. I have never seen the couples come entirely out of the water, although they frequently come so close to the shore that portions of the carapace are uncovered.”[218]

Fig. 156.—A view of the nervous system of Limulus from below. (About natural size.) After Patten and Redenbaugh.
273The carapace is represented as transparent. The appendages have been removed, but the outlines of the left entocoxites (6) have been sketched in. The positions of the abdominal appendages are indicated by the external branchial muscles (17), the branchial cartilages (19), the tendinous stigmata (18), and the abdominal endochondrites (21). In the cephalothorax (1) all the tergo-coxal and plastro-coxal muscles have been dissected away, leaving the endosternite (11) with the occipital ring exposed. One of the left tergo-proplastral muscles (4) and the left branchio-thoracic muscles (16) are represented. The longitudinal abdominal muscles are also seen. All the muscles of the right side have been omitted except the haemo-neural muscles (23), of which the last two are represented upon the left side also. At the base of the telson the flexors (29) and extensors (27) of the caudal spine are represented as cut off near their insertions. The sphincter ani (26), levator ani, and occludor ani (25), and their relations to the anus (28), are shown.
The oesophagus runs forward to the proventriculus (3). From this the intestine (20) passes posteriorly.
The brain lies upon the neural side of the endosternite, and the ventral cord (22) passes back through the occipital ring. The neural nerves are cut off, but the left haemal nerves and those from the fore-brain (12) are represented entire.
The first pair of neural nerves go to the chelicerae. The second to sixth pairs go to the next five cephalothoracic appendages, which are represented by the entocoxites (6). The seventh pair of neural nerves go to the chilaria, and the eighth pair to the operculum. The neural nerves from the ninth to the thirteenth arise from the abdominal ganglia and innervate the five pairs of gills.
From the fore-brain a median olfactory nerve (9) and two lateral ones (8) pass forward to the olfactory organ; a median eye-nerve (2) passes anteriorly and haemally upon the right of the proventriculus (3) to the median eyes; and a pair of lateral eye-nerves pass to the lateral eyes (15).
The first haemal nerve, or lateral nerve, follows the general course of the lateral eye-nerve, but continues posteriorly far back on to the neural side of the abdomen.
The haemal nerves of the hind-brain radiate from the brain to the margins of the carapace, and each one passes anterior to the appendage of its own metamere. The integumentary portions divide into haemal and neural branches, of which the haemal branches (5) are cut off. Each haemal branch gives off a small nerve which turns back toward the median line upon the haemal side of the body.
The haemal nerves of the accessory brain pass through the occipital ring to the sides of the body between the operculum and the sixth cephalothoracic appendage. The seventh innervates the posterior angles of the cephalothorax, the eighth the opercular portion of the abdomen. The next five haemal nerves arise from the five branchial neuromeres, pass out anterior to the gills to the sides of the abdominal carapace, and innervate the first five spines upon the sides of the abdomen.
The first post-branchial nerve innervates the last abdominal spine; the second post-branchial nerve and one branch of the third post-branchial innervate the posterior angles of the abdomen and the muscles of the telson; and the caudal branch of the third post-branchial nerve innervates the telson.
Intestinal branches arise from all the haemal nerves from the sixth to the sixteenth, and pass to the longitudinal abdominal muscles and to the intestine.
Cardiac nerves arise from all the haemal nerves from the sixth to the thirteenth. Six of the cardiac nerves communicate with the lateral sympathetic nerve (24), which innervates the branchio-thoracic muscles (16).
Two post-cardiac nerves arise from the first two post-branchial nerves, and passing to the haemal side anastomose with a branch from the last cardiac nerve, and innervate the extensors (27) of the telson and the epidermis behind the heart.
1, Cephalothorax; 2, median eye-nerve; 3, proventriculus; 4, tergo-proplastral muscles; 5, haemal branch of integumentary nerve; 6, entocoxites; 7, 2nd haemal nerve; 8, right olfactory nerve; 9, median olfactory nerve; 10, intestine; 11, endosternite; 12, fore-brain; 13, origin of 4th neural nerve; 14, lateral nerve; 15, lateral eye; 16, branchio-thoracic muscles; 17, external branchial muscles; 18, tendinous stigmata; 19, branchial cartilages; 20, intestine; 21, abdominal endochondrites; 22, ventral cord; 23, haemo-neural muscles; 24, lateral sympathetic nerve; 25, occludor ani; 26, sphincter ani; 27, extensors of telson; 28, anus; 29, flexors of telson; 30, lateral projections of abdomen; 31, nerves of spines; 32, external branchial muscles.

Fig. 157.—The markings on the sand made by the female Limulus when depositing eggs. Towards the lower end the round “nests” cease to be apparent, the king-crab being apparently exhausted. (From Kishinouye.) About natural size.
The developing ova and young larvae are very hardy, and in a little sea-water, or still better packed in sea-weed, will survive long journeys. In this way they have been transported from the Atlantic to the Pacific coasts of the United States, and for a time at any rate flourished in the western waters. Three barrels full of them consigned from Woods Holl to Sir E. Ray Lankester arrived in England with a large proportion of larvae alive and apparently well.
According to Kishinouye, L. longispina spawns chiefly in August and between tide-marks. “The female excavates a hole about 15 cm. deep, and deposits eggs in it while the male fertilises them. The female afterwards buries them, and begins to excavate the next hole.”[219] A line of nests (Fig. 157) is thus established which is always at right angles to the shore-line. After a certain number of nests have been formed the female tires, and the heaped up sand is not so prominent. In each “nest” there are about a thousand eggs, placed first to the left side of the nest and then to the right, from which Kishinouye concludes that the left ovary deposits its ova first and then the right. Limulus rotundicauda and L. moluccanus do not bury their eggs, but carry them about attached to their swimmerets.
The egg is covered by a leathery egg-shell which bursts after a certain time, and leaves the larva surrounded only by the 275blastodermic cuticle; when ripe it emerges in the condition known as the “Trilobite larva” (Fig. 158), so-called from a superficial and misleading resemblance to a Trilobite. They are active little larvae, burrowing in the sand like their parents, and swimming vigorously about by aid of their leaf-like posterior limbs. Sometimes they are taken in tow-nets. After the first moult the segments of the meso- and metasoma, which at first had been free, showing affinities with Prestwichia and Belinurus of Palaeozoic times, become more solidified, while the post-anal tail-spine—absent in the Trilobite larva—makes its first appearance. This increases in size with successive moults. We have already noted the late appearance of the external sexual characters, the chelate walking appendages only being replaced by hooks at the last moult.

Fig. 158.—Dorsal and ventral view of the last larval stage (the so-called Trilobite stage) of Limulus polyphemus before the appearance of the telson. 1, Liver; 2, median eye; 3, lateral eye; 4, last walking leg; 5, chilaria. (From Kingsley and Takano.)
Limulus casts its cuticle several times during the first year—Lockwood estimates five or six times between hatching out in June and the onset of the cold weather. The cuticle splits along a “thin narrow rim” which “runs round the under side of the anterior portion of the cephalic shield.”[220] This extends until it reaches that level where the animal is widest. Through this slit the body of the king-crab emerges, coming out, not as that of a beetle anteriorly and dorsally, but anteriorly and ventrally, in 276such a way as to induce the unobservant to exclaim “it is spewing itself out of its mouth.” In one nearly full-sized animal the increase in the shorter diameter of the cephalic shield after a moult was from 8 inches to 9½ inches, which is an indication of very rapid growth. If after their first year they moult annually Lockwood estimates it would take them eight years to attain their full size.
The only economic use I know to which Limulus is put is that of feeding both poultry and pigs. The females are preferred on account of the eggs, of which half-a-pint may be crowded into the cephalic shield. The king-crab is opened by running a knife round the thin line mentioned on p. 275. There is a belief in New Jersey that this diet makes the poultry lay; undoubtedly it fattens both fowls and pigs, but it gives a “shocking” flavour to the flesh of both.
CLASSIFICATION.
But five species of existing King-crabs are known, and these are grouped by Pocock into two sub-families: (i.) the Xiphosurinae, and (ii.) the Tachypleinae. These together make up the single family Xiphosuridae which is co-extensive with the Order. The following is Pocock’s classification.[221] The names used in this article are printed in italic capitals.
Order Xiphosura.
Family 1. Xiphosuridae.
Sub-Fam. 1. Xiphosurinae.
This includes the single species Xiphosura polyphemus (Linn.) (= Limulus polyphemus, Latreille), “which is said to range from the coast of Maine to Yucatan.”
Sub-Fam. 2. Tachypleinae.
Genus A. Tachypleus includes three species: (i.) T. gigas, Müll. (= Limulus gigas, Müll., and L. moluccanus, Latreille), widely distributed in Malaysia; (ii.) T. tridentatus, Leach (= L. tridentatus, Leach, and L. longispina, Van der Hoeven), extending from British North Borneo to China and Southern 277Japan; and (iii.) T. hoeveni, Pocock (= L. moluccanus, Van der Hoeven), found in the Moluccas.
Genus B. Carcinoscorpius with one species, C. rotundicauda (Latreille) (= L. rotundicauda, Latreille). It occupies a more westerly area than T. gigas or than T. tridentatus, having been recorded from India and Bengal, the Gulf of Siam, Penang, the Moluccas, and the Philippines.
With regard to the affinities of the group it is now almost universally accepted that they are Arachnids. The chief features in which they differ from other Arachnids are the presence of gills and the absence of Malpighian tubules, both being features associated with aquatic life. As long ago as 1829 Straus-Dürckheim emphasised the points of resemblance between the two groups, and although the view was during the middle of the last century by no means universally accepted, towards the end of that epoch the painstaking researches of Lankester and his pupils, who compared the King-crab and the Scorpion, segment with segment, organ with organ, tissue with tissue, almost cell with cell, established the connexion beyond doubt. Lankester would put the Trilobites in the same phylum, but in this we do not follow him. With regard to the brilliant but, to our mind, unconvincing speculations as to the connexion of some Limulus-like ancestor with the Vertebrates, we must refer the reader to the ingenious writings of Dr. Gaskell,[222] recently summarised in his volume on “The Origin of Vertebrates,” and to those of Dr. Patten in his article “On the Origin of Vertebrates from Arachnids.”[223]
Fossil Xiphosura.[224]

Fig. 159.—A., Hemiaspis limuloides, Woodw., Upper Silurian, Leintwardine, Shropshire. Natural size. (After Woodward.) B., Prestwichia (Euroöps) danae (Meek), Carboniferous, Illinois, × ⅔. (After Packard.)
Limulus is an example of a persistent type. It appears first in deposits of Triassic age, and is found again in the Jurassic, Cretaceous, and Oligocene. In the lithographic limestone of Solenhofen in Bavaria, which is of Upper Jurassic age, Limulus is common and is represented by several species. One species is known from the Chalk of Lebanon, and another occurs in the Oligocene of Saxony. No other genus of the Xiphosura 278appears to be represented in the Mesozoic and Tertiary deposits, but in the Palaeozoic formations (principally in the Upper Silurian, the Old Red Sandstone, and the Coal Measures) several genera have been found, most of which differ from Limulus in having some or all of the segments of the abdomen free; in this respect they resemble the Eurypterida, but differ from them in the number of segments. In Hemiaspis (Fig. 159, A), from the Silurian, the segments of the abdomen are divisible into two groups (mesosoma and metasoma) in the same way that they are in Eurypterids; the first six segments have broad, short terga, the lateral margins of the sixth being divided into two lobes, probably indicating the presence of two fused segments; the last three segments are narrower and longer than the preceding, and at the end is a pointed tail-spine. In Belinurus (Fig. 160) from the Carboniferous, the two regions of the abdomen are much less distinct; there are eight segments, the last three of which are fused together, and a long tail-spine. In Neolimulus, from the Silurian, there seems to be no division of the abdomen into two regions, and apparently all the segments were free. On the other hand, in Prestwichia (Carboniferous), all the segments of the abdomen, of which there appear to be seven only, were fused together (Fig. 159, B).

Fig. 160.—Belinurus reginae, Baily, Coal Measures, Queen’s Co., Ireland, × 1. (After Woodward).
In the Palaeozoic genera the median or axial part of the dorsal surface is raised and distinctly limited on each side, so presenting a trilobed appearance similar to that of Trilobites. 279In Neolimulus, Belinurus, and Prestwichia, lateral eyes are present on the sides of the axial parts of the carapace, and near its front margin median eyes have been found in the two last-named genera.
In nearly all the specimens of Palaeozoic Xiphosura[225] which have been found nothing is seen but the dorsal surface of the body; in only a very few cases have any traces of the appendages been seen,[226] but, so far as known, they appear to have the same general character as in Limulus.
Aglaspis, found in the Upper Cambrian of Wisconsin, has been regarded as a Xiphosuran. If that view of its position is correct, then Aglaspis will be the earliest representative of the group at present known. Other genera of Palaeozoic Xiphosura are Bunodes, Bunodella, and Pseudoniscus in the Silurian; Protolimulus in the Upper Devonian; and Prolimulus in the Permian.
EURYPTERIDA
CHAPTER XI
ARACHNIDA (CONTINUED)—DELOBRANCHIATA = MEROSTOMATA (CONTINUED)—EURYPTERIDA
Order II. Eurypterida.
The Eurypterida or Gigantostraca are found only in the Palaeozoic formations. Some species of Pterygotus, Slimonia, and Stylonurus have a length of from five to six feet, and are not only the largest Invertebrates which have been found fossil but do not seem to be surpassed in size at the present day except by some of the Dibranchiate Cephalopods. All the Eurypterids were aquatic, and, with the possible exception of forms found in the Coal Measures, all were marine. The earliest examples occur in the Cambrian deposits, and the latest in the Permian; but although the Eurypterids have thus a considerable geological range, yet it is mainly in the Silurian and the Old Red Sandstone that they are found, the principal genera represented in those deposits being Eurypterus, Stylonurus, Slimonia, Pterygotus, Hughmilleria, Dolichopterus, and Eusarcus. From the Cambrian rocks the only form recorded is Strabops;[227] in the Ordovician the imperfectly known Echinognathus[228] and some indeterminable fragments have alone been found. In the Carboniferous deposits Eurypterus and Glyptoscorpius occur, and the former survived into the Permian.[229]

Fig. 161.—Eurypterus fischeri, Eichw. Upper Silurian, Rootziküll, Oesel. Dorsal surface. a, Ocellus; b, lateral eye; 2–6, appendages of prosoma; 7–12, segments of mesosoma; 13–18, segments of metasoma; 19, tail-spine. (After Holm.)
The Eurypterid which is best known is Eurypterus fischeri (Figs. 161, 162), which is found in the Upper Silurian rocks at Rootziküll in the Island of Oesel (Gulf of Riga). In the Eurypterids from other deposits the chitinous exoskeleton has been altered into a carbonaceous substance, but in the specimens from Oesel the chitin is perfectly preserved in its original 285condition; and since these specimens are found in a dolomitic rock which is soluble in acid, it has been possible to separate the fossil completely from the rock in which it is embedded, with the result that the structure can be studied more easily and more thoroughly than in the case of specimens from other localities. Consequently Eurypterus fischeri[230] may, with advantage, be taken as a type of the Eurypterida.
The general form of the body (Fig. 161) is somewhat like that of a Scorpion, but is relatively broader and shorter. On the surface of many parts of the exoskeleton numerous scale-like markings are found (Figs. 162, 163).[231] The prosoma or cephalothorax consists of six fused segments covered by a quadrate carapace with its front angles rounded. This bears on its dorsal surface two pairs of eyes—large kidney-shaped lateral eyes and median ocelli (Fig. 161, b, a). The margin of the dorsal part of the carapace is bent underneath to form a rim which joins the ventral part of the carapace.
On the ventral surface of the prosoma (Fig. 162) six pairs of appendages are seen, of which only the first pair (the chelicerae) are in front of the mouth. The chelicerae are small, and each consists of a basal joint and a chela, the latter being found parallel to the axis of the body; they closely resemble the chelicerae of Limulus. The remaining five pairs of appendages are found at the sides of the elongate mouth, and in all these the gnathobases of the coxae are provided with teeth at their inner margins and were able to function in mastication, whilst the distal part of each appendage served as an organ of locomotion. The posterior part of each coxa is plate-like and is covered (except in the case of the sixth appendage) by the coxa of the next appendage 286behind. A small process or “epicoxite” is found at the posterior end of the toothed part of the coxae of the second, third, fourth, and fifth pairs of appendages. The second appendage consists of seven joints, whilst the remaining four consist of eight joints; none of these appendages end in chelae. The second, third,[232] and fourth pairs of appendages are similar to one another in structure, but become successively larger from before backwards. These three pairs are directed radially outwards; each consists of short joints tapering to the end of the limb, and bearing spines at the sides and on the under surface, and also a spine at the end of the last joint.

Fig. 162.—Eurypterus fischeri, Eichw. Upper Silurian, Rootziküll, Oesel. Restoration of ventral surface; 1–6, appendages of prosoma; m, metastoma. Immediately posterior to the metastoma is the “median process” of the genital operculum. (After Holm.)
287The fifth appendage is longer than the fourth and is directed backwards; its second and third joints are short and ring-like; the others (fourth to eighth) are long and similar to one another, each being of uniform width throughout; the last joint is produced into a spine on each side, and between these two is the movable end-spine; the other joints do not bear long spines as is the case in the three preceding pairs of appendages.
The sixth appendage is much larger and stronger than the others, and like the fifth, is without long spines. The coxa is large and quadrate; the second and third joints are short, like those of the fifth appendage; the fourth, fifth, and sixth joints are longer and more or less bell-shaped; the seventh and eighth joints are much larger than the others and are flattened.
The metastoma (Fig. 162, m) is an oval plate immediately behind the mouth; it covers the inner parts of the coxae of the sixth pair of appendages, and represents the chilaria of Limulus. But, unlike the latter, it is not a paired structure; nevertheless the presence of a longitudinal groove on its anterior part renders probable the view that it is derived from a paired organ.[233] The front margin of the metastoma is indented and toothed. On its inner side in front is a transverse plate, the endostoma, which is not seen from the exterior, since the front margin of the metastoma extends a little beyond it.
Behind the prosoma are twelve free segments, of which the 288first six form the mesosoma (Fig. 161, 7–12). The tergum on the dorsal surface of each segment is broad and short, the middle part being slightly convex and the lateral parts slightly concave; the external margin is bent under, thus forming a narrow rim on the ventral surface. The tergum of each segment overlaps the one next behind. The segments increase in breadth slightly up to the fourth segment, posterior to which they gradually become narrower.
On the ventral surface the segments of the mesosoma bear pairs of plate-like appendages, each of which overlaps the one behind like the tiles on a roof. On the posterior (or inner) surfaces of these appendages are found the lamellar branchiae, which are oval in outline (Fig. 165, d). Between the two appendages of the first pair is a median process which is genital in function; this pair are larger than the other appendages, and cover both first and second segments, the latter being without any appendages, and they represent the genital operculum of Limulus (Fig. 153, 10). The form of the operculum, more particularly of the median process, differs in the male and female. In that which is believed to be the female (Fig. 162) the median process is long, and extends beyond the posterior margin of the operculum; it is formed of two small five-sided parts at the base which are united at the sides to the two plates of the operculum; behind this is a long, unpaired part, which is pointed in front; this, together with the remaining parts, is not joined to the side-plates of the operculum, so that the latter are here separated from one another. The third part of the median process is shorter than the second, and bears at its end a pair of small pointed and diverging plates, the tips of which reach to the middle of the third plate-like appendages. On the inner side of the operculum there are, in the female, a pair of curved, tubular organs, attached to the anterior end of the median process, where they open, the free ends being closed; the function of these organs is not known, but was probably sexual.
In the male (Fig. 163, A, a) the median process is formed of two parts only, and is very short, so that the two plates of the operculum unite behind the process.
In the female a median process (Fig. 163, B) is also present between the second pair of appendages (belonging to the third segment of the mesosoma); it consists of a basal unpaired part, 289and of a pair of long pointed pieces which project on to the next segment. Just as in the case of the genital operculum the basal part is united in front to the appendages, the remainder being free, and separating the greater part of the two plate-like appendages. In the complete animal the median process of this segment is covered by the median process of the genital operculum. The remaining appendages of the female, and all the appendages behind the operculum in the male, are without any median process, and the plates of each pair unite by a suture in the middle line.

Fig. 163.—Eurypterus fischeri, Eichw. Upper Silurian. (After Holm.) A, Genital operculum of male; a, median process. B, Middle part of second appendage of the mesosoma in the female, showing the median process.
The metasoma (Fig. 161, 13–18) consists of six segments which become longer and narrower from before backwards. Each segment is covered by a ring-like sheath or sclerite, and bears no appendages. The posterior end of the last segment is produced into a lobe on each side, and between these lobes the long, narrow tail-spine arises (Fig. 161, 19).
The other genera of the Eurypterida do not differ in any important morphological respects from the form just described, 290All the genera, of which about thirteen have been recognised, are placed in one family.

Fig. 164.—Pterygotus osiliensis, Schmidt, Upper Silurian, Rootziküll, Oesel. Ventral surface. Reduced. (After Schmidt.) 1–6, Appendages of the prosoma; 7–12, mesosoma; 7, 8, genital operculum; 13–18, metasoma; 19, tail-plate; a, epistome; b, metastoma; c, coxae of sixth pair of appendages.
Fam. Eurypteridae.—The carapace varies somewhat in outline; in Slimonia it is more distinctly quadrate than in Eurypterus, whilst in Pterygotus (Fig. 164) and Hughmilleria[234] it is semi-ovoid. The lateral eyes are at the margin of the carapace in Pterygotus, Slimonia (Fig. 165, a), and Hughmilleria, but in the other genera, including the earliest form, Strabops,[235] they are on the dorsal surface at a greater or less distance from the margin.
The pre-oral appendages of Pterygotus (Fig. 164, 1) differ from those of other genera in their much greater length and in the large size of the chelae; they probably consist of a proximal joint and chelae only, although, commonly, they are represented as having a larger number of joints. Unlike Eurypterus and Pterygotus, the second pair of appendages in Slimonia (Fig. 165, 2) differ from the third, fourth, and fifth pairs in being distinctly smaller and more slender, and it is probable that they were tactile. Whilst in Eurypterus the fifth pair of appendages are larger than the three preceding pairs, and also differ from them in 291structure, in the genus Pterygotus (Fig. 164, 5) they agree closely with the second, third, and fourth pairs, and in Slimonia (Fig. 165, 5) they are nearly the same as the third and fourth pairs. The sixth pair of appendages are much larger and more powerful than the fifth pair in nearly all genera; in Stylonurus (Fig. 166), however, the sixth pair are similar to the fifth, both being greatly elongated and slender; also in Eusarcus (Drepanopterus) the sixth pair differ less from the preceding pair of appendages than is usually the case.

Fig. 165.—Slimonia acuminata, Salter. Upper Silurian. Restoration of ventral surface, × ⅑. 1–6, Appendages of prosoma; 7, 8, genital operculum; 7–12, mesosoma; 13–18, segments of metasoma; 19, tail-spine; a, lateral eye; b, metastoma, covering the inner parts of the coxae of the last pair of appendages; c, median process of genital operculum; d, branchial lamellae seen through the plate-like appendages. (After Laurie.)
In Pterygotus there is a well-developed epistome (Fig. 164, a) between the mouth and the front margin of the carapace, thus occupying the same position as the hypostome of Trilobites (p. 233). The metastoma is always well developed and forms one of the distinguishing features of the Eurypterids; in form it varies from oval in Eurypterus, to cordate in Slimonia, and lyrate in Dolichopterus.
The principal modifications seen in the genital operculum are in the form of its median process; in Slimonia this either ends in three sharp points posteriorly (Fig. 165, c), or has the form of a truncated cone; its form in Eurypterus has already been described. Glyptoscorpius differs from other Eurypterids in the possession of comb-like organs closely resembling the pectines of Scorpions. Slimonia apparently differs from other genera in that the plate-like appendages on the posterior three segments of the mesosoma do not meet in the middle line (Fig. 165, 10–12). In some forms, such as Pterygotus (Fig. 164), there is a nearly gradual decrease in the width of the segments in passing 292from the mesosoma to the metasoma; but in some others, which in this respect are less primitive, such as Slimonia (Fig. 165), the posterior five segments of the body (like those of Scorpions) are distinctly narrower and longer than the preceding segments. The long tail-spine of Eurypterus is represented in Slimonia by an oval plate produced into a spine at the end (Fig. 165, 19); whilst in some species of Pterygotus the plate is bi-lobed at the posterior end (Fig. 164, 19). In Hughmilleria the tail-spine is lanceolate.
The Eurypterids resemble the Xiphosura in many respects. In both groups the prosoma consists of at least six fused segments, and bears two pairs of eyes—one pair simple, the other grouped eyes—on the dorsal surface of the carapace. The number and position of the appendages of the prosoma in Eurypterids agree with those of Limulus. The chelicerae are closely similar in both cases. The coxae of all five pairs of legs in Eurypterids are toothed and function in mastication; similarly in Limulus all are spiny except the coxae of the last pair of legs. In both a similar epicoxite is present on the coxae. The number of joints in the legs is somewhat greater in the Eurypterids than in Limulus, and in the former none of the legs end in chelae, whereas in the latter all the walking legs, except the last, and also the first in the male, may be chelate. The metastoma of Eurypterids differs in being a large unpaired plate, but is represented in Limulus by the pair of relatively small chilaria. On the mesosoma the genital operculum and plate-like appendages with branchial lamellae are similar in both groups, but in the Eurypterids the genital operculum shows a greater development and covers the second segment, which is without plate-like appendages. A striking difference between the two groups is seen in the segments of the mesosoma and metasoma; in Eurypterids these are all free, whilst in Limulus they are fused together, but this difference is bridged over by some of the Palaeozoic Xiphosura (Fig. 159, A) in which those segments are free.

Fig. 166.—Stylonurus lacoanus, Claypole. Upper Devonian, Pennsylvania. Restoration of dorsal surface. Length nearly five feet. (After Beecher.)
The Eurypterids present a striking resemblance to Scorpions. In both groups the segments in the three regions of the body are the same in number, and the appendages of the prosoma also agree in number and position. The pre-oral appendages are chelate in both, but the second pair of appendages are chelate in the Scorpions only. In Eurypterids the coxae of the five pairs 294of legs are toothed and meet in the middle line, but in the Scorpions the coxae of the last two pairs do not meet; this difference, however, appears to be bridged over in the earliest known Scorpion—Palaeophonus,[236] from the Silurian rocks. The Eurypterids are distinguished from the Scorpions by the much greater development of the last pair of legs. The large metastoma of the former is homologous with the sternum of the Scorpion. The genital operculum is much smaller in Scorpions than in Eurypterids, and in this respect the latter agree with Thelyphonus (one of the Pedipalpi) more than with the Scorpions. The pectines are absent in the Eurypterids except in Glyptoscorpius. Instead of the lung-books of the Scorpions the Eurypterids possess branchial lamellae on the plate-like appendages; but this difference between the two groups appears to be bridged over by Palaeophonus, which was marine, and may have possessed branchial lamellae since stigmata seem to be absent.
Glyptoscorpius,[237] which is found in the Lower Carboniferous of the south of Scotland, is a form of considerable interest. It is about a foot in length, and agrees in many respects with Eurypterida, but it may be necessary to separate it from that group since it possesses pectines, and the legs end in a double claw; it cannot, however, be regarded as a link between Eurypterids and Scorpions, but must rather be considered as an offshoot from the former, since the latter group was already in existence at a much earlier period.
ARACHNIDA EMBOLOBRANCHIATA
(SCORPIONS, SPIDERS, MITES, ETC.)
CHAPTER XII
ARACHNIDA (CONTINUED)—EMBOLOBRANCHIATA—SCORPIONIDEA—PEDIPALPI
SUB-CLASS II.—EMBOLOBRANCHIATA.[238]
Order I. Scorpionidea.
Segmented Arachnids with chelate chelicerae and pedipalpi. The abdomen, which is broadly attached to the cephalothorax or prosoma, is divided into two regions, a six-jointed mesosoma and a six-jointed tail-like metasoma, ending in a poison-sting. There are four pairs of lung-books, and the second mesosomatic segment bears a pair of comb-like organs, the pectines.
The Scorpions include the largest tracheate Arachnid forms, and show in some respects a high grade of organisation. It is impossible, however, to arrange the Arachnida satisfactorily in an ascending series, for certain primitive characteristics are often most marked in those Orders which on other grounds would seem entitled to rank at the head of the group. Such a primitive characteristic is the very complete segmentation exhibited by the Scorpions. They are nocturnal animals of rapacious habit. In size they range from scarcely more than half an inch to eight inches in length. In the northern hemisphere they are not found above the fortieth parallel of latitude in the Old World, though in the New World they extend as high as the forty-fifth. A corresponding southward limit would practically include all the land in the southern hemisphere, and here the Order is universally represented except in New Zealand, South Patagonia, and the Antarctic islands.
298Fossil scorpions are rarely found. The earliest examples known occur in the Silurian rocks, and belong to the genus Palaeophonus. In the Carboniferous Eoscorpius is found, and in the Oligocene Tityus.
Much remains to be discovered with regard to the habits of scorpions, and most of the isolated observations which have been recorded lose much of their value through the uncertainty as to the species concerned. The brief accounts given by Lankester and by Pocock,[239] and the more recent and elaborate studies of Fabre,[240] are free from this defect and contain almost the only trustworthy information we possess.
All are viviparous, and the females carry the newly-hatched young on their backs. They are predaceous, feeding for the most part on insects and spiders. These are seized by the chelate pedipalps, and, if small, are simply picked to pieces by the chelicerae and devoured, but if large the tail-sting is brought into play and the victim quickly paralysed. The process of eating is a slow one, and a Cape scorpion in captivity took two hours to devour a cockroach.
In walking, scorpions carry their pedipalps horizontally in front, using them partly as feelers and partly as raptorial organs. As regards the body the attitude varies considerably. In some cases (Parabuthus, Prionurus, etc.) it is raised high upon the legs, and the “tail” or metasoma is curved forward over the back, but in others (Euscorpius) the body is held low, and the “tail” is dragged along behind, the end only being slightly curled. In the daytime they hide away under wood or stone, or in pits which they dig in the sand. Parabuthus capensis was observed to dig a shallow pit by means of its second and third ambulatory legs, resting on its first and fourth legs aided by the chelae and the metasoma. Those that hide under wood are usually found clinging to their shelter ventral side uppermost. In captivity the creatures, though supplied with water, were never observed to drink; indeed, they are characteristic inhabitants of arid steppes and parched wastes. Like most Arachnids they can endure prolonged abstinence from food.
The only sense well developed seems to be that of touch. Notwithstanding the possession of several eyes their sight is 299poor. A moving object within the range of a few inches is certainly perceived, but it has to be touched before its nature is recognised. Some writers have attributed to scorpions a keen sense of hearing, and so-called “auditory hairs” are described on the tibia of the pedipalp, but Pocock came to the conclusion that Parabuthus capensis and Euscorpius carpathicus were entirely deaf, and Lankester could obtain no indication of auditory powers in the case of Prionurus. The sense of touch is extremely delicate, and seems to reside in the hairs with which the body and appendages are more or less thickly clothed. The pectines are special tactile organs. That they are in some way related to sex seems probable from the fact that they are larger in the male and sometimes curiously modified in the female, but they appear to be of use also in determining the nature of the ground traversed by the animal, being long in such species as raise the body high on the legs, and short in those that adopt a more grovelling posture. Pocock noticed that a scorpion which had walked over a portion of a cockroach far enough for the pectines to come in contact with it immediately backed and ate it.

Fig. 167.—Buthus occitanus in the mating period. (After Fabre.)
As is the case with most poisonous animals, their ferocity has been much exaggerated; they never sting unless molested, and their chief anxiety is to slink off unobserved. The fables that they kill their young, and that when hard pressed they commit suicide by stinging themselves to death, perhaps hardly deserve serious consideration. The latter accusation is disproved by the fact that a scorpion’s poison has no effect upon itself, or even upon a closely allied species. Some writers think that in the frantic waving of the “tail,” which is generally induced by strong excitement, a scorpion may sometimes inadvertently wound itself with the sharp point of its telson.
Fabre gives a fascinating account of the habits of Buthus occitanus, which occurs in the south of France. He found 300these scorpions plentifully in arid, stony spots exposed to the sun. They were always solitary, and if two were found under the same stone, one was engaged in eating the other. Their sight is so poor that they do not recognise each other without absolute contact.
Fabre established colonies in his garden and study, providing them with suitable soil and sheltering stones. They dug holes by reducing the earth to powder by means of the three anterior pairs of legs—never using their pedipalpi in the operation—and sweeping away the débris with the tail. From October to March they ate nothing, rejecting all food offered to them, though always awake and ready to resent disturbance. In April appetite seemed to awaken, though a very trifling amount of food seemed to suffice. At that time, too, they began to wander, and apparently without any intention of returning, and they continued daily to escape from the garden enclosure until the most stringent measures were taken to keep them in. Not till they were surrounded by glass and the framework of their cages covered with varnished paper were their attempts to climb out of their prison frustrated. Fabre came to the conclusion that they took at least five years to attain their full size.

Fig. 168.—The “promenade à deux” of Buthus occitanus. (After Fabre.)
His most interesting observations were concerned with their mating habits, in connection with which he noted some extraordinary phenomena. After some very curious antics, in which the animals stood face to face (Fig. 167) with raised tails, which they intertwined—evidently with no hostile intention—they always indulged in what Fabre calls a “promenade à deux,” hand in hand, so to speak, the male seizing the chelae of the female with its own, and walking backwards, while the female followed, usually without any reluctance. This promenade occupied an hour or more, during which the animals turned several times. At length, if in the neighbourhood of a suitable stone, the male would dig a hole, without for a moment entirely quitting its hold of the female, 301and presently both would disappear into the newly-formed retreat.
After mating, the male was often devoured by the female. Moreover, after any combat with an enemy, such as a Lycosa or a Scolopendra, it appeared to be de rigueur to eat the vanquished, and on such occasions only was any considerable amount of food consumed.
The scorpions were not, however, anxious to fight, greatly preferring to retire if possible; but when incited to combat, their sting was quickly fatal to any mature insect, to spiders and to centipedes. Curiously enough, however, insect larvae, though badly wounded, did not succumb to the poison. Newly-hatched scorpions mounted on the mother’s back, where they remained motionless for a week, entirely unfed. They then underwent a moult, after which they were able to forage for themselves.
External Structure.
The chitinous plates of the prosoma are fused to form a carapace. Six segments are clearly indicated by the six pairs of appendages, but, though the development of Scorpio affords little direct evidence of the fact, there is reason to believe that there once existed a pre-cheliceral segment,[241] as has been clearly proved in the case of the spiders. An embryonic pregenital segment has also been recognised. The six prosomatic appendages are those proper to the Arachnida, being the chelicerae, pedipalpi, and four pairs of ambulatory legs. The mesosoma, which is broadly attached to the prosoma, comprises six segments, indicated ventrally by the genital operculum, the pectines, and the four pairs of pulmonary stigmata. The last of the broad abdominal segments, which tapers abruptly, belongs to the metasoma, which also comprises six segments, and is succeeded by the post-anal spine or sting.
Prosoma.—Near the middle of the carapace are two median eyes, and on its antero-lateral borders are usually to be found groups of smaller eyes, numbering from two to five. All the eyes are simple. There is a difference, however, in their development, the median eyes being diplostichous, or involving two layers of hypoderm, while the lateral eyes are monostichous, and pass through a stage strikingly like the permanent condition of the 302eyes of Limulus. The arrangement of various slight longitudinal ridges on the dorsal surface of the carapace is of systematic importance. On the ventral surface, just in front of the genital operculum, is a sternum, never large, and sometimes barely visible. Its shape and size constitute one of the principal family characteristics.

Fig. 169.—Buthus occitanus. A, Dorsal view; B, ventral view. (After Kraepelin.)
Mesosoma.—The dorsal plates or terga are distinct, and are connected by soft chitin with their corresponding sterna.
Beneath the second abdominal segments are borne the “pectines” or comb-like organs. In their structure four portions are distinguishable, an anterior lamella or shaft attaching them to the body, a middle lamella, the teeth, and the fulcra, a series of small chitinous pieces intercalated between the bases of the movable teeth.
303Beneath the third, fourth, fifth, and sixth segments are the paired openings of the lung-sacs.
Metasoma.—The first segment is usually and the remainder are invariably enclosed in complete chitinous rings and show considerable variations in their comparative size and shape, and in the arrangement of the ridges and keels with which they are usually furnished. The post-anal segment is more or less globular at its base, constituting a “vesicle,” and terminates in a fine curved point, the “aculeus,” perforated for the passage of the delicate poison-duct. With the abdomen fully extended the point is directed downward, but in the attitude of attack or defence, when the “tail” is carried horizontally over the back, the sting points forward in the neighbourhood of the animal’s head.

Fig. 170.—A, Diagram of a Scorpion’s leg; 1, coxa; 2, trochanter; 3, femur; 4, patella; 5, tibia; 6, protarsus; 7, tarsus; p.s, pedal spur; t.s, tibial spur. B, Fourth tarsus of Palamnaeus swammerdami; l, lateral lobe. (After Pocock.)
Appendages.—The three-jointed chelicerae are powerful and chelate. The first joint is small, but the second is strongly developed and bears at its anterior end on the inner side a projection which forms the immovable finger of the chela. The third joint, or movable finger, is articulated on the outer side, and both fingers are armed with teeth whose arrangement is useful in distinguishing the species. The pedipalpi consist of six joints. The coxa is small and has an inwardly directed lamella which assists in feeding. The trochanter is also a small joint, bearing, normally at right angles to the longitudinal axis, the powerful humerus or femur. Then follows the brachium or tibia, again directed forward, and the last two joints form the chela or “hand,” the terminal joint or movable finger being on the outer side as in the chelicerae. In systematic determination special attention is given to the “hand.” In some forms the upper surface is uniformly rounded, while in others a “finger-keel” divides it into two flattish surfaces almost at right angles. The biting edges of the fingers are usually furnished with rows of minute teeth arranged characteristically in the different genera. The ambulatory legs are seven-jointed, though, unfortunately, authors are not agreed upon the nomenclature of the joints. Kraepelin[242] names them coxa, trochanter, femur, tibia, and three-jointed tarsus, and Simon[243] agrees with him. Pocock’s names[244] 304are coxa, trochanter, femur, patella, tibia, protarsus, and tarsus, and it is certainly convenient that each joint should have a separate name, but it must be borne in mind that the tibia of different authors is not always the same joint. Special attention must be directed to the three terminal joints, which furnish highly important characteristics. The tibia (in Pocock’s sense) is sometimes provided with a “tibial spur” at its lower distal extremity. From the soft arthrodial membrane between the protarsus and tarsus may proceed one or more dark-tipped claw-like spurs, the “pedal spurs.” The terminal joint (tarsus of Pocock) is variously furnished with hairs and teeth, and always ends in a pair of well-developed movable claws beneath which a much reduced and sometimes almost obsolete third claw is distinguishable. The tarsus generally projects in a “claw-lobe” over the base of the superior claws, and sometimes lateral lobes are present. The first and second coxae have triangular maxillary lobes directed towards the mouth. The third and fourth coxae are fused together on each side, and those on one side are separated from those on the other by the sternum. In other respects the four pairs of legs are usually similar.
Internal Anatomy.
The alimentary canal is a fairly uniform tube, nowhere greatly dilated. The very small mouth leads into a small suctorial chamber, and this is connected by a narrow oesophagus, which pierces the cerebral nerve-mass, with a slightly dilated portion which receives the ducts of the first pair of gastric 305glands, often called salivary glands. The succeeding portion in the prosoma receives four or five more pairs of ducts from the well-developed gastric glands. In the rapidly narrowing first metasomatic segment the intestine receives one or two pairs of Malpighian tubes, and thence proceeds to the anus, situated ventrally in the last segment.
The vascular system is of the usual Arachnid type, the heart being a seven-chambered dorsal longitudinal vessel lying in a pericardium, with which it communicates by seven pairs of valvular ostia. Lankester[245] has demonstrated several pairs of superficial lateral veins connecting two deep-seated ventral venous trunks with the pericardium. The lung-books are, so to speak, pushed in to dilatations of these trunks, so that some of the lateral veins carry blood newly aerated by the lung-books directly to the pericardium.
The nervous system is not greatly concentrated except in the prosoma, where there is a single ganglionic mass which innervates not only the whole prosoma but the mesosoma as far as the first and sometimes the second pair of lung-books. There are two mesosomatic ganglia, variously situated in different genera, and each metasomatic segment has its ganglion.
The generative organs are more or less embedded in the gastric glands. There are two testes, each composed of a pair of intercommunicating tubules, and connected by a common vas deferens with the generative aperture, which is furnished with a double protrusible intromittent organ. A pair of vesiculae seminales and a pair of accessory glands are also present. The female possesses a single ovary, consisting of a median and two lateral tubules, all connected by cross branches.
In addition to the external sclerites a free internal skeletal plate, situated in the prosoma between the alimentary canal and the nerve-cord, furnishes convenient fulcra for muscular attachment. It is known as the “endosternite.”
Brauer[246] has made the most complete study of the development of Scorpio, and two of the most interesting of his conclusions may be mentioned here. He has shown the lung-books to be derived from gills borne on mesosomatic appendages. Moreover he found in the embryo five pairs of segmental ducts—in 306segments 3–6 and 8—and demonstrated that those of segment 5 persisted, though without external aperture, as coxal glands, and those of segment 8 as the genital ducts.
Classification.
More than 350 species of scorpions have been described, but many of these are “doubtful,” and probably the number of known forms may be put at about 300. These are divided by Kraepelin[247] into six families and fifty-six genera. The best indications of the family of a scorpion are to be found in the shape of the sternum, the armature of the tarsi, and the number of the lateral eyes, while assistance is also to be derived from the shape of the stigmata and of the pectines, and from the absence or presence of a spine beneath the aculeus.
The six families are: Buthidae, Scorpionidae, Chaerilidae, Chactidae, Vejovidae, and Bothriuridae.
Fam. 1. Buthidae.—Sternum small and generally triangular. Tibial spurs in the third and fourth legs. Generally a spur beneath the aculeus. Lateral eyes three to five in number.
There are two sub-families: Buthinae and Centrurinae.
The Buthinae, which possess a tibial spur, comprise fourteen genera, most of them Old World forms. The principal genera are Buthus, which contains about 25 species, and Archisometrus with 20 species. One genus only, Ananteris, is South American, and it includes only a single species. The genus Uroplectes, with 16 species, is almost entirely African.
The Centrurinae, without tibial spur, are New World scorpions, though Isometrus europaeus (maculatus) is cosmopolitan. The principal genera are Tityus with 30 species, Centrurus with 13, and Isometrus with 6.
Fam. 2. Scorpionidae.—Sternum broad and pentagonal, with sides approximately parallel. No tibial spur, but a single pedal spur. Generally three lateral eyes.
Nearly a hundred species of Scorpionidae have been described, distributed among fifteen genera. The following sub-families are recognised: Diplocentrinae, Urodacinae, Scorpioninae, Hemiscorpioninae, and Ischnurinae.
307The Diplocentrinae have a spur under the aculeus. They form a small group of only eight species. The principal genus, Diplocentrus, is entirely Neotropical, but Nebo has a single Old World representative in Syria.
The Urodacinae, with the single genus Urodacus, are Australian scorpions. As in the next sub-family, there are rounded lobes on the tarsi, but there is only a single keel on the “tail,” and the lateral eyes are two in number. Six good and three doubtful species are recognised.
The Scorpioninae are Asiatic and African forms, and are recognised by the tarsi having a large lobe on each side, by the convex upper surface of the “hand,” by the presence of two median keels on the “tail,” and by the possession of three lateral eyes. Palamnaeus (Heterometrus) has sixteen species in the Indian region. There are about thirty species of Opisthophthalmus, all natives of South Africa. Pandinus includes about ten species, but there are only two species of the type genus Scorpio, S. maurus and S. boehmei.
The sub-family Hemiscorpioninae was formed for the reception of the single Arabian species Hemiscorpion lepturus. Its most striking characteristic is the cylindrical vesicle of the tail in the male.
The Ischnurinae differ from the Scorpioninae chiefly in the absence of the tarsal lobes, the presence of a well-marked finger-keel, and the generally more depressed form of the body and hand. In the opinion of some authors they should be separated from the Scorpionidae as a distinct family, the Ischnuridae. There are more than twenty species, divided among six genera. The type genus Ischnurus has only the single species I. ochropus. There are eight species of Opisthacanthus, which has representatives in Africa and America.
Fam. 3. Chaerilidae.—Sternum pentagonal with median depression or “sulcus” rounded posteriorly. Two pedal spurs. Stigmata circular. Two lateral eyes with a yellow spot behind the second. Pectines very short.
This small family has the single genus Chaerilus with but seven species, natives of the Oriental region.
Fam. 4. Chactidae.—Two pedal spurs. Two lateral eyes (or, rarely, no eyes) but without yellow spot. Characteristic dentition on movable finger of “hand.”
308There are three sub-families, Megacorminae, Euscorpiinae, and Chactinae.
The Megacorminae include but a single Mexican form, Megacormus granosus. There is a single toothed keel under the “tail,” and all the under surface is spiny. There is a row of long bristles under the tarsus.
In the Euscorpiinae the upper surface of the hand is divided into two surfaces almost at right angles by a strong finger-keel. This is a small group of about six species found in the Mediterranean region. The two genera are Euscorpius and Belisarius.
The Chactinae are without any marked keel on the hand. The scorpions of this sub-family are found in equatorial South America and the West Indies, where there are more than twenty species divided about equally between the four genera Chactas, Broteas, Broteochactas, and Teuthraustes.
Fam. 5. Vejovidae.—No tibial, but two pedal spurs. A single row of hairs or papillae under the tarsus. Sternum generally broader than long. Elongate stigmata, and three lateral eyes.
Seven of the eight genera of this family include only American forms, the principal genus being Vejovis, with about ten species. The genus Scorpiops, however, belongs to the Indian region and numbers more than ten species.[248]
Fam. 6. Bothriuridae.—Sternum much reduced and sometimes hardly visible, consisting of two slight, nearly transverse bars.
Of the seven genera of this family one, Cercophonius, is Australian. The other six genera include some dozen South American forms, Bothriurus having four species.
Order II. Pedipalpi.
Arachnids with non-chelate, two-jointed chelicerae, powerful pedipalpi, and four pairs of legs, of which only the last three are ambulatory, the first being used as tactile organs. The cephalothorax is usually covered by an undivided carapace, but the pedunculated abdomen is segmented. Respiration is by lung-books.
The Pedipalpi are a little-known group of animals of nocturnal habits. Though rarely seen they are widely distributed, being found in India, Arabia, the greater part of Africa, and Central 309and South America. They are of ancient origin, a fossil genus, Graeophonus, of the Tarantulidae (Phrynidae, see p. 312), occurring in the Carboniferous strata in North America. They live under stones and bark, and in caves, where, when disturbed, they seek safety in crannies in the rock.
Little is known of their habits, but they are believed to feed chiefly upon insects. The female Tarantula carries the developing eggs, somewhat after the manner of the Chernetidea (see p. 434), in a bag beneath the abdomen, the under surface of which becomes concave and dome-like during the period of gestation.[249]

Fig. 171.—Thelyphonus, diagrammatic ventral view; about natural size. c, Coxal joint of pedipalp; g, generative opening; p, pedipalp; sp, spiracles; st, sternal plates; 1, 2, 3, 4, ambulatory legs. (After Pickard-Cambridge.)
External Structure.—The external features which the members of this Order have in common are the segmented pediculate abdomen (9 to 12 segments), the two-jointed non-chelate chelicerae, the antenniform first pair of legs, and the presence of two pairs of lung-book stigmata beneath the abdomen. The constituent families differ so much in outward form that they must be dealt with separately.
The Thelyphonidae or “Whip Scorpions” (see p. 312) have a long-oval carapace bearing well-developed eyes, two in front, and a group of three or five on either side some distance behind. The pedipalpi are chelate, and have their basal joints fused beneath the mouth, being thus incapable of any masticating motion.
The first legs are six-jointed, and have multi-articulate tarsi; the others are seven-jointed, and their tarsi, in some species at least, are tri-articulate. The abdomen consists of two portions, a wide nine-jointed pre-abdomen and a short narrow three-jointed post-abdomen, to which a filiform tail is articulated. Beneath the cephalothorax, between the coxae of the legs, is a distinct sternal plate in two portions (Fig. 171). The first abdominal ventral plate is largely developed, and covers two segments. Behind it are the median 310genital opening and two pulmonary stigmata, while the other stigmata are behind the second ventral plate, which corresponds to the third abdominal segment. On the last abdominal segment there are often two or four light-coloured spots called “ommatoids,” and considered by some authors to be organs of sight. Laurie, however (vide infra), thinks it more probable that they are olfactory in function.
The Schizonotidae (see p. 312) have a two-jointed carapace, and do not possess more than two eyes. There is a short unjointed tail-piece.
In the Tarantulidae (Phrynidae) the whole body is much flattened and extended laterally, the undivided carapace being reniform, and broader than long. The long non-chelate pedipalps have their basal joints free and movable, and there are several sternal plates. There are nine abdominal tergal plates, the last three diminishing rapidly in size, and the last plate covering a button-like terminal portion of the abdomen. The first abdominal ventral plate is largely developed, as in the Thelyphonidae, and the genital orifice and pulmonary stigmata are in the same situation as in that group. The Tarantulidae have glutinous glands in the first abdominal segment which are capable of spinning a few irregular threads.
In the whole group paired circular depressions are conspicuous dorsally on all the abdominal segments. These indicate the points of attachment of the dorso-ventral muscles.
Internal Structure.—The anatomy of the Pedipalpi has been very inadequately studied. Disconnected notes on various points of structure have been published by various morphologists, but no complete investigation has yet been made of the internal organs. This is largely due to the difficulty of obtaining material, and the bad state of preservation of the internal parts of such specimens as have been available for dissection.
The following points have been made out in the anatomy of Thelyphonus.[250]
The alimentary canal commences after the mouth with a pharynx which, though not dilated, is furnished with sucking muscles. It then narrows into an oesophagus which passes through the nerve-mass, and afterwards dilates to form the mid-gut, which immediately gives off two large lateral diverticula 311which extend backwards, each having five lobes. There are also two median diverticula which proceed from the ventral surface and pass through the endosternite. The abdominal portion of the canal is entirely concealed by the great “liver” mass which communicates with it by four paired ducts in the anterior part of the abdomen. Behind the fourth abdominal segment the gut is narrow till it expands in the seventh segment into an hour-glass-shaped stercoral pocket which, according to Laurie, is a portion of the mesenteron.
The excretory organs are the Malpighian tubes and the coxal glands. The former are generally described as entering the anterior portion of the stercoral pocket, but according to Laurie they pass along its ventral surface, attached to it by connective tissue, and really enter at the posterior end. The coxal glands are well developed, and lie beneath the endosternite, opening near the first coxae.
The nervous system is much concentrated and of the usual Arachnid type. The median abdominal nerve has a ganglion towards its extremity, supplying, according to Bernard,[251] the muscles which move the tail. The heart is extremely long, and varies little in width. It has nine pairs of ostia[252]—two in the thorax and seven in the abdomen. The generative glands are paired, and in the male there are large seminal vesicles. In the most ventral portion of the abdominal cavity lies a remarkable asymmetrically-situated gland, the “stink-gland.” It consists of a number of secretory tubules communicating with two elongated sacs, one of which lies beneath the nerve-cord, and therefore medially, while the other lies far to the left. Their ducts proceed to the anus or its vicinity.
The caudal organs, or white spots which, as already mentioned, are usually found on the last of the three post-abdominal segments of Thelyphonus, are of doubtful function. They have been variously explained as the stink-gland orifices, and as organs sensitive to light (“ommatoids”). Laurie[253] was unable to find any pore in this region, nor was there any of the pigment so characteristic of organs of sight. The histological structure indicated a sense-organ rather than a gland, but the use of these organs is entirely conjectural.
312Classification.—The order Pedipalpi is divided into three families—Thelyphonidae, Schizonotidae and Tarantulidae. The first two are considered by some authors to form a sub-order, Uropygi, or tailed Pedipalpi, while the Tarantulidae constitute the remaining sub-order Amblypygi, the members of which are tailless.
Fam. 1. Thelyphonidae.[254]—This family comprises nine or more genera, differing chiefly in the position of the eyes, the structure of the genital operculum, the armature of the pedipalps, and the presence or absence of “ommatoids” in the anal segment.
The three following genera are among those most likely to be met with. Two ommatoids are present in each.
Thelyphonus has a spine on the second ventral plate, and a deep median impression on the male genital operculum, which is, however, absent from that of the female. There are about fifteen known species of this genus, inhabiting Southern Asia and the East Indies.
Typopeltis has ridges running forward from the lateral eyes. The middle third of the female operculum is raised and deeply impressed in the middle. This genus is represented in China and Japan. Mastigoproctus has a short and stout coxal apophysis of the pedipalp, without a tooth on its inner side. It is found in Mexico, Brazil, and the West Indies. Other genera are Thelyphonellus (Demerara), Labochirus (Ceylon), Hypoctonus (Burma), Mimoscorpius (Philippines), Uroproctus (Assam), Abalius (New Guinea), without ommatoids, and Tetrabalius (Borneo), with two pairs of ommatoids.
Fam. 2. Schizonotidae (= Tartaridae).—This family contains only two genera, Schizonotus (= Nyctalops, Pickard-Cambridge, nom. preocc. Aves) and Trithyreus[255] (= Tripeltis, Thorell, nom. preocc. Reptilia). They are very small, pale-coloured forms (about 5 mm. in length), found in Burma and Ceylon.
Fam. 3. Tarantulidae, better known as Phrynidae. Pocock has shown that Fabricius established the genus Tarantula from the species T. reniformis in 1793, while there is no earlier record of Olivier’s Phrynus, established for the same species, than Lamarck’s citation of it in 1801. The family is 313divided into three sub-families, Tarantulinae, Phrynichinae, and Charontinae.
(i.) The Tarantulinae are new-world forms, represented by three genera, Tarantula, Acanthophrynus (Phrynopsis), and Admetus (Heterophrynus), in Central and South America and the West Indies.
(ii.) The Phrynichinae belong to the Old World, being found in Africa, India, and Ceylon. Phrynichus, Titanodamon and Nanodamon are genera of this sub-family.
(iii.) The Charontinae are natives of South-East Asia and the Pacific Islands. There are five genera and eight species.
CHAPTER XIII
ARACHNIDA EMBOLOBRANCHIATA (CONTINUED)—ARANEAE—EXTERNAL STRUCTURE—INTERNAL STRUCTURE.
Order III. Araneae.
(ARANEIDA,[256] ARANEINA.)
Arachnida breathing by tracheae and “lung-books.” Cephalothorax and pedicellate abdomen, the latter usually soft, and only very rarely showing any traces of segmentation. Two-jointed non-chelate chelicerae, the distal joint bearing the orifice of a poison-gland. The tarsal joint of the male pedipalp develops a sexual organ. The abdomen is furnished with spinning mammillae.
The true Spiders can readily be distinguished from allied Arachnid groups, with which they are often popularly confounded, by the presence of a narrow constriction or “waist” between the cephalothorax and abdomen, and of a group of “spinnerets” or external spinning organs beneath the hind portion of the body. Thus the so-called “Harvest-spider” or “Harvestman” is clearly not a Spider, for there is no constriction of its body into two parts, nor does it possess any spinnerets. It belongs to the Phalangidea. The same considerations will exclude the “Red Spider” of popular nomenclature, which must be referred to the Acarina or Mites.
The Araneae, even as at present known, form a very extensive and widely-distributed order of animals. Compared with certain insect orders, they have received little attention from the collector, 315and the number of known forms is certain to be very largely increased. They form an extremely compact and natural group, for though, within the order, there is an infinite variety of detail, their uniformity in essential points of structure is remarkable, and they are sharply marked off from the neighbouring groups of Arachnida.

Fig. 172.—Epeira angulata. ♀.
It is perhaps unfortunate that the obtrusiveness of particularly unattractive specimens of the race has always caused spiders to be regarded with more or less aversion. This prejudice can hardly fail to be modified by a wider acquaintance with these animals. There are certainly few groups which present points of greater interest in respect to their adaptation to special modes of life and the ingenuity displayed in the construction of their nests and the ensnaring of their prey.
Spiders are wingless, yet they may often be observed travelling through the air. They are air-breathing, yet many are amphibious in their habits, and one species at least spends the greater part of its existence beneath the surface of the water. On land they may be found in all imaginable localities which admit of the existence of that insect life on which they depend for food.
External Structure.—The spider’s body consists of two portions, the cephalothorax and the abdomen.
Cephalothorax.—Looked at dorsally (Fig. 173), the cephalothorax is generally seen to have a depression near the middle, the “median fovea,” and from this certain lines, the “radial striae,” radiate towards the sides. These depressions indicate the attachment of internal muscles.
The head region or “caput” lies in front of the foremost of the radial striae, and is often clearly marked off from the thorax, and different from it in elevation. It bears the eyes, which, in the great majority of spiders, are eight in number. Many, however, are six-eyed, while in rare cases the number is reduced to four (Tetrablemma, see p. 404), or even to two (Nops, see p. 395). 316The number, relative size, and particular arrangement of these eyes are of considerable systematic importance. Their disposition varies very greatly, but it is generally possible to regard them as forming two transverse rows, an anterior and a posterior, each possessing a pair of median and a pair of lateral eyes.

Fig. 173.—Diagrammatic dorsal view of a Spider. ch, Chelicera; f, median fovea; n, normal marking; o, ocular area; p, pedipalp; st, stria. (The dotted line should reach the radial marking on the cephalothorax.)
In many spiders all the eyes have a dorsal aspect, but in some groups (Attidae, Lycosidae) the prevailing arrangement is to have the anterior eyes directed forwards and the posterior upwards. In other spiders, again, a dorsal view may only show the eyes in profile, all having their axes directed forwards or sideways, or they may be mounted on turrets, and thus command a wide range of view. The rows are described as straight, “procurved” (with the convexity backwards), or “recurved” (with the convexity forwards). Thus, in Fig. 177, the anterior row is slightly, and the posterior row considerably “recurved.”
Sometimes there is a marked difference in the colour of the eyes, two or more being black, while the remainder are pearly white. In other cases they are homogeneous, either of the black or the white type. Simon considers the black eyes to be diurnal and the white nocturnal, but the evidence for this is indirect and not altogether satisfactory. The portion of the caput occupied by the eyes is often alluded to as the “ocular area.” The space between the ocular area and the chelicerae, well shown in Fig. 177, is known as the “clypeus.” It is usually more or less vertical, but in the Aviculariidae (see p. 386) it is horizontal and dorsal.
The under surface of the cephalothorax is protected by the “sternum” or “plastron,” a large plate of variable shape, usually notched at either side for the reception of the legs, and having in front a small plate, generally hinged, but sometimes soldered to it, known as the “labium.” This has no homology with the labium of insects, but is a true sternite, more correctly described as “pars labialis sterni.”
The labium and the maxillary lobes of the palpi more or less 317conceal the under surface of the caput. The shape of the sternum and of the labium, and the contour and degree of inclination towards one another of the maxillae, are important considerations in the taxonomy of Spiders.

Fig. 174.—Diagrammatic ventral view of a Spider. Cephalothorax—l, Labium; m, maxilla; p, paturon of chelicera; st, sternum; u, unguis of chelicera. Abdomen—a.t, Anal tubercle; c, colulus; ep, epigyne; s, stigma; sp, spinnerets; tr, tracheal opening.
The appendages of the cephalothorax, which are the chelicerae or jaws, the pedipalpi or feelers, and the four pairs of ambulatory legs, will be treated separately.
Pedicle.—The chitinous investment of the narrow stalk which unites the thorax with the abdomen is for the most part thin and flexible, with only slight indurations of various patterns on the dorsal surface, where it is in most cases more or less protected by the forwardly-projecting abdomen. Beneath, it is usually quite membranous, guarded only by a sort of collar formed by the raised border of the anterior portion of the abdomen at the point of insertion. In some Spiders, however (Dysderidae), there is a posterior sternal plate, the “plagula,” closely corresponding with the labium in front, which partly embraces the pedicle. In Hermippus (Zodariidae) the plagula is detached from the sternum, and is succeeded posteriorly by two smaller paired plates.
Abdomen.—The abdomen differs remarkably in shape in the different groups of Spiders. In some families the prevailing shape is more or less globular, and in others cylindrical, while it may be diversified to almost any extent by prominences or spines. Ordinarily no sign of segmentation is observable, but in Liphistius it is covered dorsally by seven well-marked chitinous plates.
In most Spiders the integument of the abdomen is uniformly soft and flexible all over, but it is not rare to find portions of it thickened and hardened to form “scuta.” In the Gasteracanthinae and the Phoroncidinae there is a great dorsal scutum armed with spines, while in several families there are species characterised by the possession of a smooth dorsal scutum; and in some a ventral scutum is present.

Fig. 175.—Spider profiles. 1, Poltys ideae; 2, Phoroncidia 7–aculeata; 3, Ariamnes flagellum; 4, Stegosoma testudo; 5, Formicinoides brasiliana.
That these scuta are sometimes indicative of an obsolete segmentation would seem likely from the study of the remarkable species, Tetrablemma medioculatum (Fig. 176), described by Pickard-Cambridge, from Ceylon. In addition to large dorsal and ventral scuta, the sides and posterior extremity are guarded by smaller scuta, the disposition of which is well seen in the figure.

Fig. 176.—Tetrablemma medioculatum, much enlarged. A, Posterior view; B, profile, showing the scuta. (After Cambridge.)
The normal smooth abdomen presents dorsally no very striking features. In species of variegated coloration there is very generally noticeable a median dentated band (Fig. 173), the “normal marking” of some writers, which would appear to have some correlation with the underlying dorsal vessel. Beneath the abdomen are to be seen the orifices of the breathing and genital organs, the spinnerets, and the anal aperture upon its tubercle.
The breathing organs are, as will be explained later, of two kinds, lung-books and tracheae. The great majority of Spiders possess only two lung-books, and their transverse, slit-like openings (“stigmata” or “spiracles”) may be seen on either side of the anterior part of the abdomen. Where, as in the Theraphosae, there are four lung-books, the second pair open by similar slits a short distance behind the first. According to Bertkau, pulmonary sacs are entirely lacking in the genus Nops.
The tracheae generally debouch by a single median stigma towards the posterior end of the abdomen, just in front of the spinnerets. This opening clearly results from the fusion of two stigmata, which in some species retain their paired arrangement.
On a level with the openings of the anterior lung-books or pulmonary sacs there is usually observable a slight transverse 319ridge, the epigastric fold (Fig. 174), and in the centre of this is the genital opening. This is never visible until after the last moult, and in the male is always a simple inconspicuous aperture. This is also the case with the females of some groups (Theraphosae, Filistatidae, Dysderidae, etc.), but in most cases there is a more or less complicated armature, the “epigyne,” the special design of which is of great specific value. In its simplest form it is merely a plate, usually of dark colour, with one or two apertures (Fig. 174, ep), but in some families, notably the Epeiridae, it is more complicated, and is furnished with a hooked median projection, the “ovipositor” (“clavus” of Menge), which is often absurdly like a petrified elephant’s trunk in miniature.
The abdomen also presents on its under surface, usually towards the posterior end or apex, a group of finger-like mammillae or spinnerets. They are normally six in number, two superior (or posterior), two median, and two inferior (or anterior). The number is reduced, in most of the Theraphosae, to four, while a few spiders possess only a single pair of spinnerets. These organs are described more fully on p. 325.
A small papilla, the “colulus” (Fig. 174, c), is often observable, projecting between the anterior spinnerets. The “anal tubercle” (Fig. 174, a.t), on which the vent is situated, terminates the abdomen, and is generally in close juxtaposition with the posterior spinnerets.
Appendages.—The cephalothoracic appendages are the chelicerae, the pedipalpi, and the four pairs of ambulatory legs. Those of the abdomen are the mammillae or spinnerets.
Chelicerae.—These are two-jointed appendages, articulated immediately below or in front of the clypeus. They are the “mandibles” of many authors, but there is good reason for believing that they are not homologous with the mandibles of Insects. There is little agreement, moreover, with regard to the names given to the two joints of which they consist. The term “falx,” often applied to the basal joint, is much more appropriate to the sickle-like distal joint. Base and fang are tolerably satisfactory, or we may avoid ambiguity by adopting the terms “paturon” and “unguis” suggested by Lyonnet.[257]
The paturon is a stout joint of more or less cylindrical or conical shape. The unguis (the “crochet” of Simon) is hook-like, 320and can generally be folded back upon the paturon, which often presents a groove for its reception. The Theraphosid spiders are distinguished from all others by the fact that the plane of action of the chelicerae is vertical and longitudinal. The paturon projects forward in a line parallel with the axis of the body, and its distal end can be raised or depressed, but not moved laterally; while the unguis in action has the point directed downwards, and, at rest, is applied to the under surface of the paturon.
In other spiders the patura hang more or less vertically, and while to some extent mobile in all directions, their principal motion is lateral, and the ungues have their points directed towards each other in action, and are applied to the inner surfaces of the patura in repose. The plane of action in this case is also more or less vertical, but transverse.

Fig. 177.—Front view of Textrix denticulata. × about 10. 1, Caput; 2, eyes; 3, paturon; and 4, unguis of chelicera.
The paturon is always extremely hard and strong. In Theraphosae of burrowing habits the distal end is furnished with a group of powerful teeth, the “rastellus.” The groove for the reception of the unguis is often guarded on one side or on both by rows of teeth, the arrangement of which is frequently an important specific character. The inner anterior border is also often furnished with a group of stiff hairs or bristles. This powerful joint is of use in crushing and expressing the fluids of insects pierced by the ungues.
The crescent-shaped unguis is tapering and smooth, except for the presence, on the posterior surface, of one or two feebly dentated ridges. Near its free extremity there is a small orifice leading to the poison reservoir and gland.
In the genus Pholcus (see p. 401) the chelicerae may almost be regarded as chelate, the unguis being met by a spiny projection from the inner anterior border of the paturon.
Rostrum.—On examining a spider, even under a dissecting microscope, it will not be easy at first to discover the mouth. Indeed, Lyonnet had almost come to the conclusion that Spiders, like some Myrmelionid larvae, imbibed the juices of their prey by way of the mandibles, before he found the orifice and gave a remarkably accurate description of the adjacent parts.
If a specimen be placed on its back, and the labium raised 321while the chelicerae are pushed forward, no orifice is visible, but on careful examination it will be found that what appears to be a thick and fleshy labium is, in reality, two organs. The labium is thin and flat, and closely opposed to its upper surface is a somewhat flattened cone. This is the “rostrum,” and when it is separated from the labium the buccal orifice is disclosed. In a few spiders (Archeidae) in which the chelicerae are far removed from the mouth, the rostrum is tolerably conspicuous, but in most it is so hidden as to have escaped the observation of the great majority of observers. Schimkewitsch considers it homologous with the labrum of insects, but Simon thinks that it represents all the insect mouth-parts reduced to an exceedingly simple form. It is more probable that a beak consisting of a simple labrum and labium was a primitive Arachnid characteristic. If the rostrum be removed and its inner (or posterior) surface examined, a lance-shaped chitinous plate, the “palate,” becomes visible. It is furrowed down the middle by a narrow groove, which is converted into a tube for the passage of fluids when the rostrum is opposed to the labium.

Fig. 178.—Pedipalp of Tegenaria domestica ♂, × 5. 1, Coxa; 2, maxilla; 3, trochanter; 4, femur; 5, patella; 6, tibia; 7, tarsus; 8, palpal organ.
Pedipalpi.—The pedipalpi are extremely leg-like feelers, and are six-jointed, the metatarsal joint of the ambulatory legs being absent. The joints, therefore, are the coxa, trochanter, femur, patella, tibia, and tarsus (Fig. 178).[258]
In the Theraphosae the coxa resembles that of the ambulatory leg, but in other spiders it is furnished, on the inner side, with a blade-like projection, the “maxilla” (Fig. 178). The shape of the maxillae and the degree of their inclination towards the labium are of considerable taxonomic importance. The inner border of the maxilla is furnished with a tuft of hairs, 322which assist in retaining the juices expressed by the chelicerae, and its anterior border presents a cutting edge with a finely dentated ridge called the “serrula.”
In the female, and in the immature male, the remaining joints differ little from those of the legs, except that the tarsal joint is either clawless or has a single claw, which is generally smooth, and is never much dentated.
At the last moult but one the male pedipalp appears tumid at the end, and after the last moult the tarsus is seen to have developed a remarkable copulatory apparatus, the “palpal organ,” comparatively simple in some families, but in others presenting an extraordinary complexity of structure.

Fig. 179.—Diagram of palpal organ. 1, Tarsus; 2, bulb; 3, receptaculum seminis; 4, its aperture; 5, style; 6, haematodocha; 7, alveolus; 8, tibia.
Palpal Organs.—Externally the essential parts of the palpal organ are three, the “haematodocha,” the “bulb,” and the “style.” The spines and projections, or “apophyses,” which often accompany the palpal organ proper, are of secondary importance, and in many spiders are entirely absent; nor is their function when present at all clear; but the infinite variety of design which they exhibit, and their singular uniformity in all the males of a species, render them of the utmost value as specific characteristics.
The “haematodocha” is the portion of the palpal organ attached to the tarsus, and often received into an excavation, the “alveolus,” on its under surface. It is a fibro-elastic bag, in its normal collapsed state usually somewhat spirally disposed round the base of the following portion, the “bulb.”
The bulb is generally the most conspicuous portion of the organ, and is a sub-globular sac with firm, though often semi-transparent, integument. Its base rests upon the haematodocha, and its apex is produced, often spirally, to a point which bears the seminal orifice. This external opening leads into a coiled 323tube within the bulb, ending in a blind sac, the “receptaculum seminis,” which projects into the haematodocha; and it is the aperture by which the sperm both enters and leaves the organ. How the sperm is conveyed to the receptaculum was long a matter for speculation, after the belief in a direct communication between the generative glands and the pedipalpi had been abandoned. The process has been actually observed in the case of a few spiders, which have been seen to deposit their sperm on a small web woven for the purpose, and then, inserting the styles of their palpal organs into the fluid, to suck it up into the receptacula seminis. This is probably the usual method of procedure, though it may be true, as some have asserted, that the palp is sometimes applied directly to the genital orifice.
The receptaculum and its tube being thus charged with sperm, it is the function of the haematodocha to eject it by exerting pressure on its base. For this purpose the haematodocha is in communication with the cavity of the tarsus, from which, in copulation, it receives a great flow of blood, and becomes greatly distended. Bertkau believes that he has detected very minute pores (meatus sanguinis) communicating between the haematodocha and the receptaculum, and allowing some of the blood-plasma from the former to mingle with the semen, but this appears to be very doubtful.
The Legs are uniformly eight in number, and are seven-jointed, the joints, counting from the body, being the coxa, trochanter, femur, patella, tibia, metatarsus, and tarsus.[259] In a few cases, through the presence of false articulations, i.e. rings of softer chitin, this number appears to be exceeded. Some of the Palpimanidae (see p. 398) were at first thought to have only six joints on their anterior legs, but the tarsus is present, though very small.
In the case of most spiders, the legs take a general fore and aft direction, the first pair being directed forwards, the second forwards or laterally, and the third and fourth backwards. In the large group of “Crab-spiders” (Thomisidae), and in many of the Sparassinae, all the legs have a more or less lateral direction, and the spider moves with equal ease forwards, backwards, or sideways. The legs are usually more or less thickly clothed 324with hairs, but in some genera the clothing is so sparse that they appear glossy, while in others they have a positively shaggy appearance. Stouter hairs or “bristles” are often present, and some of the joints are also often furnished with “spines,” which in many cases are erectile.
The tarsi of all spiders are furnished with terminal claws, usually three in number, though in some families (Drassidae, Thomisidae, etc.) there are only two. The two principal claws are paired and usually dentated, though the number of their teeth may be unequal. The third claw, when present, is always smaller, median, and inferior.

Fig. 180.—Spider tarsi. 1, Tarsus of Epeira showing three claws and supplemental serrate hairs (a); 2, tarsus of a Thomisid Spider, with two claws; 3, 3a, lateral and dorsal view of tarsus of an Attid Spider, showing scopula at b.
In many spiders of climbing habits the place of the third claw is taken by a remarkable tuft of club-like hairs termed a “scopula” (Fig. 180, b), by means of which they are able to cling to smooth surfaces where claws would be able to obtain no hold. In some species there is a special false articulation—the “onychium”—at the end of the tarsus to bear the claws.
In the Cribellatae the metatarsus is always furnished with a comb-like organ, the “calamistrum,” correlated with an extra spinning apparatus, the “cribellum,” but this will be dealt with when we reach the systematic portion of the subject.
The general direction taken by the legs, the comparative length of the different joints, their armature of hairs, bristles, and spines, and the number and conformation of the tarsal claws, are points of great importance in the classification of Spiders.
325Under considerable magnification the legs of all Spiders exhibit a number of minute organs, arranged with absolute uniformity throughout the Araneae, and known as the “lyriform organs.” They consist of little parallel ridges of thickened chitin, the slit between them being covered by thinner chitin. They are eleven on each leg, and are distributed near the distal extremities of each of the first six joints. Their function is unknown, though some authors consider them to be organs of hearing.

Fig. 181.—Spinnerets of Epeira diademata. A, Ventral view of Epeira; B, spinnerets magnified; C, profile.
The Spinnerets are normally six in number, and, except in rare instances, are placed beneath the abdomen, near its apex and immediately in front of the anal tubercle. Their arrangement varies greatly, but they can generally be recognised as comprising three pairs, a posterior (or superior) pair, a median pair, and an anterior (or inferior) pair.
In nearly all the Theraphosae the anterior pair are absent, while the posterior spinnerets are largely developed. In the Palpimanidae only the anterior spinnerets are present. When all six are found, the usual arrangement is in the form of a rosette, the median spinnerets being hidden by the others in repose, but this disposition is widely departed from. In Hahnia (Agelenidae), for instance, they are ranged in a transverse row at the end of the abdomen, the posterior spinnerets occupying the extremities of the row, and the median ones the centre.
These spinnerets are highly mobile appendages, and additional play is given to their action by the presence of articulations, much resembling the “false” joints sometimes found on the legs, on the posterior and anterior pairs. They are always at least bi-articulate, and sometimes present three or four joints. They are movable turrets on which are mounted the “fusulae” or projections where the tubes from the 326spinning glands open. These are often very numerous, especially in the orb-weaving spiders, where the spinning powers are most highly developed. They consist of two portions, a cylindrical or conical basal part, succeeded by a very fine, generally tapering tube.
In some spiders the fusulae are all much alike, but usually a few very much larger than the rest are noticeable under the microscope, and these are often alluded to as “spigots.” The smaller ones are also divisible into two kinds, a few short conical fusulae being noticeable amongst the much more numerous cylindrical tubes. We shall treat of the functions of the various fusulae later (see pp. 335 and 349).
Simon remarks that though the battery of fusulae is most complicated in those spiders which possess the greatest spinning powers, it is by no means among them that extremely long spinnerets are developed. The posterior spinnerets of some of the Hersiliidae are of great length, but these spiders spin very little except in forming their egg-cocoons.

Fig. 182.—A, Spinnerets of Amaurobius similis ♀. Much enlarged. a, Anus; cr, cribellum; i.s, inferior spinneret; m.s, median spinneret; s.s, superior spinneret. B, Part of the 4th leg of the same Spider, showing the calamistrum (ca) on the metatarsus.
In addition to the six spinnerets, and just in front of them, there is to be found in some spiders an extra spinning organ in the form of a double sieve-like plate, the “cribellum.” This is always correlated with a comb of curved bristles on the metatarsi of the fourth pair of legs, the “calamistrum.” Such importance is assigned to these organs by Simon, that the Araneae Veraeare divided by him according to whether they are present or absent, into Cribellatae and Ecribellatae. This is probably an exaggerated view of the importance of these organs, and the 327spiders possessing them certainly do not seem to form a natural group.
Stridulating Organs.—When Arthropod animals are capable of producing a sound, the result is nearly always obtained by “stridulation,” that is, by the friction of two rough surfaces against each other. The surfaces which are modified for this purpose form what is called a “stridulating organ.” Such organs have been found in three very distinct Spider families, the Theridiidae, the Sicariidae, and the Aviculariidae. Hitherto they have only been observed in three positions—either between the thorax and abdomen, or between the chelicerae and the pedipalpi, or between the pedipalpi and the first legs.
In the Sicariidae and the Aviculariidae, the sounds have been distinctly heard and described. Those produced by the Theridiidae would appear to be inaudible to human ears.

Fig. 183.—Stridulating apparatus of Steatoda bipunctata, ♂. Much enlarged. A, Ridged and toothed abdominal socket; B, striated area on the cephalothorax; C, profile of the Spider, × 5.
Westring[260] was the first to discover (1843) a stridulating organ in the small Theridiid spider Asagena phalerata. The abdomen, where the pedicle enters it, gives off a chitinous collar, which projects over the cephalothorax, and has the inner surface of the dorsal part finely toothed. When the abdomen is raised and depressed, these teeth scrape against a number of fine striae on the back of the posterior part of the cephalothorax. A similar organ has been since found in various allied spiders, of which the commonest English species is Steatoda bipunctata. In this group it is generally possessed by the male alone, being merely rudimentary, if present at all, in the female.
In 1880 Campbell[261] observed that in some of the Theridiid Spiders of the genus Lephthyphantes, the outer surface of the 328chelicera and the inner surface of the femur of the pedipalp were finely striated at the point, where they were rubbed together when the palps were agitated, but though the appropriate motion was frequently given, he could hear no sound.

Fig. 184.—Chilobrachys stridulans in stridulating attitude. After Wood-Mason. Natural size.
Meanwhile the noise produced by a large Theraphosid spider in Assam (Chilobrachys stridulans) had attracted attention, and its stridulating apparatus was described in 1875 by Wood-Mason.[262] The sound resembled that obtained by “drawing the back of a knife along the edge of a strong comb.”
Subsequently certain Sicariid spiders of a genus confined to the southern hemisphere were heard to produce a sound like the buzzing of a bee by the agitation of their palps, and both sexes were found to possess a very perfect stridulating organ, consisting of a row of short teeth on the femur of the pedipalp, and a striated area on the paturon of the chelicera.
Pocock has recently discovered that all the large kinds of Theraphosidae in the countries between India and New Zealand are, like Chilobrachys, provided with a stridulating organ. In these spiders also it is between the palp and the chelicera, and consists of a row of teeth or spines constituting a “pecten,” and a series of vibratile spines or “lyra,” but whereas in Chilobrachys and its near relations the lyra is on the palp and the pecten on the paturon, in other spiders the positions are reversed. The lyra is a very remarkable organ, consisting of club-shaped, often feathery bristles or spines, which lie parallel to the surface to which they are attached, and which is slightly excavated for their reception.
Lastly, many African Theraphosids possess a similar organ, 329not between the palp and the chelicera, but between the palp and the first leg.
Various suggestions have been hazarded as to the use of these organs, but they partake largely of the nature of conjecture, especially in connexion with the doubt as to the possession of a true auditory organ by the Araneae. They may be summarised as follows. The Theridiid spiders are among those which show most indication of auditory powers, and the stridulating organs, being practically confined to the male, may have a sexual significance. Chilobrachys stridulates when attacked, assuming at the same time a “terrifying attitude,” and its stridulating organ may serve the purpose attributed to the rattle of the rattlesnake, and warn its enemies that it is best let alone. If this be the case, there is no need that it should itself hear the sound, and, indeed, there is no evidence that the Aviculariidae possess the power of hearing. In the inoffensive stridulating Sicariid spiders the sounds could hardly serve this purpose, and the presence of the organ in both sexes, and in immature examples, precludes the idea that its function is to utter a sexual call. Instead of trying to escape when disturbed, the spider starts stridulating, and Pocock suggests that the similarity of the sound produced to the buzzing of a bee may be calculated to induce its enemies to leave it in peace.
Internal Anatomy.
Alimentary System.—The alimentary canal of the Spider is divided into three regions, the “stomodaeum,” the mid-gut or “mesenteron,” and the hind-gut or “proctodaeum.”
The Stomodaeum consists of the pharynx, the oesophagus, and the sucking stomach. As we have said, the mouth is to be found between the rostrum and the labium. It opens into the pharynx, the anterior wall of which is formed by a chitinous plate on the inner surface of the rostrum, sometimes called the palate. As the inner surfaces of the rostrum and labium are practically flat, the cavity of the pharynx would be obliterated when they are pressed together, were it not for a groove running down the centre of the palate, which the apposed labium converts into a tube, up which the fluids of the prey are sucked. In the Theraphosidae there is a corresponding groove on the inner surface of the labium.
330At the top of the pharynx, which is nearly perpendicular, the canal continues backwards and upwards as a narrow tube, the oesophagus, passing right through the nerve-mass, which embraces it closely on all sides, to the sucking stomach. At the commencement of the oesophagus is the opening of a gland, probably salivary, which is situated in the rostrum.

Fig. 185.—Diagram showing the anatomy of the cephalothorax of a Spider. The right alimentary diverticulum has been removed. a, Aorta; c, left diverticulum with secondary caeca; e, endosternite; oes, oesophagus, descending to the mouth; s, sucking stomach; sh, dorsal shield of sucking stomach.
We now reach the sucking stomach, which occupies the centre of the cephalothorax. It is placed directly over a skeletal plate, the “endosternite” (Fig. 185, e), to which its lower surface is connected by powerful muscles, while its upper wall is protected by a hard plate or “buckler,” which is similarly attached to the roof of the cephalothorax in the region of the “fovea media.” The walls of the stomach are not themselves muscular, but by the contraction of the muscles above mentioned its cavity is enlarged, and fluids from the pharynx are pumped up into it.
The canal thus far is lined by chitin, like the exterior of the body, and forms a sort of complicated mouth-apparatus.
The Mesenteron lies partly in the cephalothorax and partly in the abdomen. The thoracic portion, shortly behind the sucking stomach, sends forward on either side a large branch or “diverticulum,” from each of which five secondary branches or “caeca” are given off (Fig. 185). Of these the anterior pair sometimes join, thus forming a complete ring; but usually, though adjacent, they remain distinct. The other four pairs of caeca curve downwards, protruding into the coxae of the legs, where they often terminate, but sometimes (Epeira) they continue their curve until they meet, though they never fuse, under the nerve-mass. Behind the origin of the diverticula the mesenteron continues as a widish tube, and shortly passes through the pedicle and enters the abdomen, where, curving slightly upwards, it proceeds along the middle line till it ends in the proctodaeum.
331In the abdomen it is surrounded by a large gland, the so-called liver, and is dilated at one spot (Fig. 186) to receive the ducts from this gland. The fluid elaborated by this large abdominal gland has been shown to have more affinity with pancreatic juice than with bile.
The Proctodaeum consists of a short rectum, from the dorsal side of which protrudes a large sac, the “stercoral pocket.” At its origin, the rectum receives the openings of two lateral tubes which reach it after ramifying in the substance of the liver. These have been called “Malpighian tubules,” but their function is unknown. Loman[263] has shown that they open into the mid-gut and not into the rectum, and there is reason to believe that true Malpighian tubules homologous to those of Insecta are absent in Arachnida, where their place seems to be taken by the coxal glands, which are considered to be the true excretory organs. In most spiders they open near the third coxae. Like the stomodaeum, the proctodaeum has a chitinous lining.
Vascular System.—The earlier investigations on the circulation of the blood in Spiders were made by direct observations of the movements of the blood corpuscles through the more or less transparent integuments of the newly-hatched young. Claparède’s[264] results were arrived at by this method. It is invaluable for demonstrating roughly the course taken by the blood, but in these immature spiders the blood-system has not attained its full complexity, and other methods of research have shown the spider to possess a much more elaborate vascular system than was at first suspected.
The tubular heart lies along the middle line in the anterior two-thirds of the abdomen, sometimes close up against the dorsal wall, but occasionally at some little distance from it, buried in the substance of the liver. It is a muscular tube with three pairs of lateral openings or “ostia,” each furnished with a simple valve which allows the entrance, but prevents the exit, of the blood. It is contained in a bag, the “pericardium,” into which the ostia open. Both heart and pericardium are kept in place by a complicated system of connective tissue strands, by which they are anchored to the dorsal wall of the abdomen. Eight 332arteries leave the heart, the principal one, or “aorta,” plunging downward and passing through the pedicle to supply the cephalothorax. Besides this, there is a caudal artery at the posterior end, and three pairs of abdominal arteries, which proceed from the under surface of the heart, and the ramifications of which supply, in a very complete manner, the various organs of the abdomen. The heart is not divided up into compartments. The anterior aorta passes through the pedicle, above the intestine, and presently forks into two main branches, which run along either side of the sucking stomach, near the front of which they bend suddenly downwards and end in a “patte d’oie,” as Causard[265] expresses it—a bundle of arteries which proceed to the limbs (Fig. 185). Where the downward curve begins, a considerable artery, the mandibulo-cephalic, runs forward to supply the chelicerae and the head region. We have omitted certain minor branches from the main trunks which supply the thoracic muscles. The nerve-mass receives fine vessels from the “patte d’oie.”

Fig. 186.—Diagram of a Spider, Epeira diademata, showing the arrangement of the internal organs, × about 8. 1, Mouth; 2, sucking stomach; 3, ducts of liver; 4, so-called Malpighian tubules; 5, stercoral pocket; 6, anus; 7, dorsal muscle of sucking stomach; 8, caecal prolongation of stomach; 9, cerebral ganglion giving off nerves to eyes; 10, sub-oesophageal ganglionic mass; 11, heart with three lateral openings or ostia; 12, lung-sac; 13, ovary; 14, acinate and pyriform silk-glands; 15, tubuliform silk-gland; 16, ampulliform silk-gland; 17, aggregate or dendriform silk-glands; 18, spinnerets or mammillae; 19, distal joint of chelicera; 20, poison-gland; 21, eye; 22, pericardium; 23, vessel bringing blood from lung-sac to pericardium; 24, artery.
There are no capillaries, but the blood is delivered into the tissues and finds its way, by irregular spaces or “lacunae,” into certain main venous channels or “sinuses.” There are three such 333in the cephalothorax, one median and the others lateral, considerably dilated in front, in the region of the eyes, and connected by transverse passages. By these the blood is brought back through the pedicle to the lung-books. In the abdomen also there are three main sinuses, two parallel to one another near the lower surface, and one beneath the pericardium. These likewise bring the blood to the lung-books, whence it is conducted finally by pulmonary veins (Fig. 186) back to the pericardial chamber, and thus, by the ostia, to the heart.
The Spider’s blood is colourless, and the majority of the corpuscles are “amoeboid,” or capable of changing their shape.
Generative System.—The internal generative organs present no great complexity, consisting, in the male, of a pair of testes lying beneath the liver, and connected by convoluted tubes, the “vasa deferentia,” with a simple aperture under the abdomen, between the anterior stigmata.
The ovaries are hollow sacs with short oviducts which presently dilate to form chambers called “spermathecae,” which open to the exterior by distinct ducts, thus forming a double orifice, fortified by an external structure already alluded to as the “epigyne.” The eggs project from the outer surface of the ovary like beads, connected with the gland by narrow stalks, and it was not at first clear how they found their way into the interior cavity, but it has been ascertained that, when ripe, they pass through these stalks, the empty capsules never presenting any external rupture.
The palpal organs have already been described. The spermatozoa, when received by them, are not perfectly elaborated, but are contained in little globular packets known as “spermatophores.”
Nervous System.—The Spider’s central nervous system is entirely concentrated in the cephalothorax, near its floor, and presents the appearance of a single mass, penetrated by the oesophagus. It may, however, be divided into a pre-oesophageal portion or brain, and a post-oesophageal or thoracic portion.
The brain supplies nerves to the eyes and chelicerae, while from the thoracic mass nerves proceed to the other appendages, and through the pedicle to the abdomen. The walls of the oesophagus are closely invested on all sides by the nerve-sheath or neurilemma.
Sense-Organs.—Spiders possess the senses of sight, smell, and 334touch. Whether or not they have a true auditory sense is still a matter of doubt. Since sounds are conveyed by vibrations of the air, it is never very easy to determine whether responses to sounds produced near the animal experimented upon are proofs of the existence of an auditory organ, or whether they are only perceived through the ordinary channels of touch. In any case, the organs of hearing and of smell have not yet been located in the Spider. M‘Cook considers various hairs scattered over the body of the spider to be olfactory, but from Gaskell’s researches upon allied Arachnid groups it would seem that the true smelling organ is to be sought for in the rostrum.
Eyes.—Spiders possess from two to eight simple eyes, the external appearance and arrangement of which have already been briefly explained. They are sessile and immovable, though often so placed as to command a view in several directions. In structure they are essentially like the ocelli of Insects. Externally there is a lens, succeeded by a mass of transparent cells, behind which is a layer of pigment. Then come the rods and cones of the retina, to which the optic nerve is distributed. A comparison of this with the arrangement in the Vertebrate eye will show a reversal of the positions of the retina and the pigment-layer. The lens is part of the outside covering of the animal, and is cast at the time of moulting, when the spider is temporarily blind. It is stated, however, that the eyes do not all moult simultaneously. There is often a considerable difference between the various eyes of the same spider, especially with regard to the convexity of the lens and the number of rods and cones.
Though most spiders possess eight eyes, the number is sometimes smaller, and in some groups of eight-eyed spiders two of the eyes are sometimes so reduced and degenerate as to be practically rudimentary. As might be expected, Cave-spiders (e.g. Anthrobia mammouthia) may be entirely sightless.
Touch.—The sense of touch would appear to be extremely well developed in some spiders, and there is reason for believing that the Orb-weavers, at all events, depend far more upon it than upon that of sight.
Among the hairs which are distributed over the spider’s body and limbs, several different forms may be distinguished, and some of them are undoubtedly very delicate sense-organs of probably tactile function.
335Spinning Glands.—Spiders vary greatly in their spinning powers. Some only use their silk for spinning a cocoon to protect their eggs, while others employ it to make snares and retreats, to bind up their prey, and to anchor themselves to spots to which they may wish to return, and whence they “drag at each remove a lengthening chain.”
All these functions are performed by the silk-glands of the Orb-weavers, and hence it is with them that the organs have attained their greatest perfection. We may conveniently take the case of the common large Garden-spider, Epeira diademata. The glands occupy the entire floor of the abdomen. They have been very thoroughly investigated by Apstein,[266] and may be divided into five kinds.

Fig. 187.—Spinning glands. A, Aciniform; B, tubuliform; C, piriform gland.
On either side of the abdomen there are two large “ampullaceal” glands debouching on “spigots,” one on the anterior, and one on the middle spinneret; there are three large “aggregate” glands which all terminate on spigots on the posterior spinneret; and three “tubuliform” glands, two of which have their orifices on the posterior, and one on the middle spinneret. Thus, in the entire abdomen there are sixteen large glands, terminating in the large fusulae known as spigots. In addition to this there are about 200 “piriform” glands whose openings are on the short conical fusulae of the posterior and anterior spinnerets, and about 400 “aciniform” glands which debouch, by cylindrical fusulae, on the middle and posterior spinnerets. Thus there are, in all, about 600 glands with their separate fusulae in the case of Epeira diademata.
The great number of orifices from which silk may be emitted has given rise to the widespread belief that, fine as the Spider’s line is, it is woven of hundreds of strands. This is an entire misconception, as we shall have occasion to show when we deal with the various spinning operations.
A few families are, as has already been stated, characterised by the possession of an extra spinning organ, the cribellum, and 336the orifices on this sieve-like plate lead to a large number of small glands, the “cribellum glands.”
Respiratory Organs.—Spiders possess two kinds of breathing organs, very different in form, though essentially much alike. They are called respectively “lung-books” and “tracheae.” The Theraphosae (and Hypochilus) have four lung-books, while all other spiders, except Nops, have two. Tracheae appear to be present almost universally, but they have not been found in the Pholcidae.
The pulmonary stigmata lead into chambers which extend forwards, and which are practically filled with horizontal shelves, so to speak, attached at the front and sides, but having their posterior edges free. These shelves are the leaves of the lung-book. Each leaf is hollow, and its cavity is continuous, anteriorly and laterally, with the blood-sinus into which the blood from the various parts of the Spider’s body is poured.
The minute structure of the leaf is curious. Its under surface is covered with smooth chitin, but from its upper surface rise vast numbers of minute chitinous points whose summits are connected to form a kind of trellis-work. The roof and floor of the flattened chamber within are connected at intervals by columns. The pulmonary chamber usually contains from fifteen to twenty of these leaves, and the two chambers are always connected internally between the stigmata.
The tracheae are either two or four (Dysderidae, Oonopidae, Filistatidae) in number, and their stigmata may be separate or fused in the middle line. Each consists of a large trunk, projecting forwards, and giving off tufts of small tubes which lose themselves among the organs of the abdomen, but do not ramify. In the tracheae of Argyroneta[267] a lateral tuft is given off immediately after leaving the stigma, and another tuft proceeds from the anterior end. Histologically the main trunk of the trachea is precisely like the general chamber of the pulmonary sac, and differs greatly from the trachea of an insect.
Cephalothoracic Glands.—In addition to the generative glands and the so-called “liver” which occupy so large a portion of the abdomen, there are, in Spiders, certain glandular organs situated in the cephalothorax which call for some notice. These are the coxal glands and the poison-glands.
337The COXAL GLANDS are two elongated brownish-yellow bodies, situated beneath the lateral diverticula of the stomach, and between it and the endosternite. They present four slight protuberances which project a short distance into the coxae of the legs. The glands appear to be ductless, but their function is thought to be excretory. They were first observed in the Theraphosae.
All Spiders possess a pair of POISON-GLANDS, connected by a narrow duct with a small opening near the extremity of the fang of the chelicerae. The glands are sac-like bodies, usually situated in the cephalothorax, but sometimes partially (Clubiona) or even entirely (Mygale) in the patura, or basal joints of the chelicerae. Each sac has a thin outer layer of spirally-arranged muscular and connective tissue fibres, and a deep inner epithelial layer of glandular cells. The cavity of the gland acts as a reservoir for the fluid it secretes. The virulence of the poison secreted by these glands has been the subject of much discussion, and the most diverse opinions have been held with regard to it. The matter is again referred to on p. 360.
CHAPTER XIV
ARACHNIDA EMBOLOBRANCHIATA (CONTINUED)—ARANEAE (CONTINUED)
HABITS—ECDYSIS—TREATMENT OF YOUNG—MIGRATION—WEBS—NESTS—EGG-COCOONS—POISON—FERTILITY—ENEMIES—PROTECTIVE COLORATION—MIMICRY—SENSES—INTELLIGENCE—MATING HABITS—FOSSIL SPIDERS
EARLY LIFE OF SPIDERS.
Ecdysis or Moulting.—Spiders undergo no metamorphosis—that is to say, no marked change of form takes place, as is so often the case among Insects, in the period subsequent to the hatching of the egg. This fact, by the by, is a great trouble to collectors, as it is generally extremely difficult, and sometimes quite impossible, to identify immature specimens with certainty.
But although unmistakably a spider as soon as it leaves the egg, the animal is, at first, in many respects incomplete, and it is only after a series of moults, usually about nine in number, that it attains its full perfection of form.
Until the occurrence of its first moult it is incapable of feeding or spinning, mouth and spinning tubes being clogged by the membrane it then throws off. It is at first pale-coloured and less thickly clothed with hairs and spines than it eventually becomes, and the general proportions of the body and the arrangement of the eyes are by no means those of the adult in miniature, but will be greatly modified by unequal growth in various directions. It speedily, however, attains its characteristic shape and markings, and after one or two ecdyses little alteration is to be noticed, except increase in size, until the final moult, when the spider at length becomes sexually mature.
339The first moult takes place while the newly-hatched spider is still with the rest of the brood either in or close to the “cocoon” or egg-bag. M‘Cook[268] thus describes the conclusion of the operation in the case of Agelena naevia:—
“While it held on to the flossy nest with the two front and third pairs of legs, the hind pair was drawn up and forward, and the feet grasped the upper margin of the sac-like shell, which, when first seen, was about half-way removed from the abdomen. The feet pushed downwards, and at the same time the abdomen appeared to be pulled upward until the white pouch was gradually worked off.”
The later moults are generally accomplished by the spider collecting all its legs together and attaching them with silk to the web above, while the body, also attached, hangs below. The old skin then splits along the sides of the body, and the animal, by a series of violent efforts, wriggles itself free, leaving a complete cast of itself, including the legs, suspended above it. For a day or two before the operation the spider eats nothing, and immediately upon its completion it hangs in a limp and helpless condition for a quarter of an hour or so, until the new integument has had time to harden. It is not unlikely that the reader has mistaken these casts for the shrivelled forms of unlucky spiders, and has had his sympathies aroused, or has experienced a grim satisfaction, in consequence—an expenditure of emotion which this account may enable him to economise in future.
Limbs which the animal has accidentally lost are renewed at the time of moulting, though their substitutes are at first smaller than those they replace. On the other hand, the struggle to get rid of the old skin sometimes results in the loss of a limb, and the spider is doomed to remain short-handed until the next ecdysis.
Until the last moult the generative apertures, which are situated under the anterior part of the abdomen, are completely sealed up. Their disclosure is accompanied, in the case of the male, by a remarkable development of the last joint of each pedipalp, which becomes swollen and often extremely complicated with bulbs, spines, and bristles. A mature male spider may at once be distinguished by the consequent knobbed appearance of its palps; and the particular form they assume is highly characteristic of the species to which the spider belongs.
340The number of moults, and the intervals at which they occur, no doubt vary with different species. In the case of Argiope aurelia, Pollock[269] has found that the female moults nine times after leaving the cocoon, the first ecdysis occurring after an interval of from one to two months, according to the abundance or scarcity of food. The subsequent intervals gradually increase from about a fortnight to something over three weeks.
Behaviour of the Newly-hatched Spider.—The mode of life of a spider just freed from the cocoon will of course vary greatly according to the Family to which it belongs.
The Epeiridae are the builders of the familiar wheel or orb-web. Spiders of this Family usually remain together on friendly terms for a week or more after leaving the nest. Most of the time they are congregated in a ball-like mass, perhaps for the sake of warmth, but upon being touched or shaken they immediately disperse along the multitudinous fine lines which they have spun in all directions, to reassemble as soon as the panic has subsided. Such a ball of the yellow and black offspring of the large Garden-spider, Epeira diademata, is no uncommon sight in the early autumn, and the shower of “golden rain” that results from their disturbance is not likely to be forgotten if it has ever been observed by the reader. This harmonious family life only continues as long as the young spiders are unable to feed—a period which, in some of the larger species, is said to extend to ten days or a fortnight.
Individual life then commences, and each member of the dispersed group sets up housekeeping on its own account, constructing at the first attempt a snare in all respects similar, except in size, to those of its parent.
Of course the young Spiders have not migrated far, and a bush may frequently be seen covered by the often contiguous nets of the members of a single brood. This, as Dr. M‘Cook thinks, is the true explanation of some of the cases of “gregarious spiders” which Darwin[270] and other naturalists have occasionally described, though social spiders exist (see Uloborus, p. 411).
Very similar habits obtain among the Theridiidae, or line-weaving spiders, a familiar example of which is the pretty little Theridion sisyphium, whose highly-irregular snare may be found on any holly bush during the summer months.

Fig. 188.—A, Pardosa sp. ♀, with young on the abdomen; B, young Pardosa detached; C, outline of the Spider with young removed. (From the living specimen.)
The Lycosidae, or Wolf-spiders, which chase their prey instead of lying quietly in ambush to ensnare it, are exceedingly interesting in their treatment of their young. The cocoon, or bag of eggs, is carried about on all their expeditions, attached beneath the abdomen, or held by the jaws, and the young spiders, on escaping from it, mount on the mother’s back, and indulge vicariously in the pleasures of the chase from this point of vantage. The empty egg-bag is soon discarded, but the brood continues to ride on the mother’s back for about a week, dismounting only to follow her as she enters her little silk-lined retreat in the ground.
During this time they appear to require no food, but they at length begin to disperse, the mother gently but firmly removing such individuals as are disposed to trespass upon her maternal solicitude longer than she considers desirable.
Many young spiders of various Families proceed immediately to seek new hunting-grounds by the aid of the wind, and become for the time being diminutive aeronauts. This habit was observed by the earliest British araneologist, Martin Lister,[271] as long ago as 1670, and has been alluded to by many writers since his time.
The topmost bar of an iron railing in spring or early autumn will generally be found peopled with minute spiders, and if the day be fair and the wind light, the patient observer may be rewarded by a curious and interesting sight.
The spider seeks the highest spot available, faces the wind, and straightens its legs and body, standing, so to speak, upon its toes, its abdomen with its spinning tubes being elevated as much as possible. Streamers of silk presently appear from the spinnerets 342and float gently to leeward on the light current of air. The spider has no power to shoot out a thread of silk to a distance, but it accomplishes the same result indirectly by spinning a little sheet or flocculent mass which is borne away by the breeze.

Fig. 189.—Young Spider preparing for an aerial voyage. (After Emerton.)
When the streaming threads pull with sufficient force the animal casts off, seizes them with its legs, and entrusts itself to the air, whose currents determine the height to which it is carried and the direction of its journey. The duration, however, is not quite beyond the spider’s control, at all events in calm weather, for it can furl its sail at will, hauling in the threads “hand-over-hand,” and rolling them up into a ball with jaws and palps.
This curious ballooning habit of young Spiders is independent of the particular family to which they belong, and it is remarkable that newly-hatched Lycosidae and Aviculariidae, whose adult existence is spent entirely on or under the ground, should manifest a disposition to climb any elevated object which is at hand.
The “Gossamer,” which so puzzled our forefathers, is probably no mystery to the reader. It is, of course, entirely the product of Spider industry, though not altogether attributable to the habit of ballooning above described. Only a small proportion of gossamer flakes are found to contain spiders, though minute insects are constantly to be seen entangled in them. They are not formed in the air, as was supposed long after their true origin was known, but the threads emitted by multitudes of spiders in their various spinning operations have been intermingled and carried away by light currents of air, and on a still, warm day in spring or autumn, when the newly-hatched spider-broods swarm, the atmosphere is often full of them.
They rise to great heights, and may be carried to immense distances. Martin Lister relates how he one day ascended to the highest accessible point of York Minster, when the October air teemed with gossamer flakes, and “could thence discern them yet exceeding high” above him. Gilbert White describes a shower, at least eight miles in length, in which “on every side, as the observer turned his eyes, he might behold a continual succession 343of fresh flakes falling into his sight, and twinkling like stars as they turned their sides toward the sun.” The ascent of a hill 300 feet in height did not in the least enable him to escape the shower, which showed no sign of diminution.
The mortality among very young spiders must be exceedingly great; indeed, this is indicated by the large number of eggs laid by many species, an unfailing sign of a small proportion of ultimate survivors. We shall have, by and by, to speak of some of their natural enemies, but apart from these their numbers are sadly reduced by the rigours of the weather, and appreciably also by their tendency to cannibalism. A thunderstorm will often destroy a whole brood, or they may perish from hunger in the absence of an adequate supply of insects minute enough for their small snares and feeble jaws. In the latter case they sometimes feed for a time on one another, and it is even said that two or three of a brood may be reared on no other food than their unfortunate companions.
The large and handsome Garden-spider, Epeira diademata, has been known, when well fed, to construct six cocoons, each containing some hundreds of eggs, and some species are even more fertile, while their adult representatives remain stationary, or even diminish in number.
Spider-Webs.—Some account has already been given of the external and internal spinning organs of Spiders. Within the body of the animal the silk is in the form of a gummy fluid; and this, being emitted in exceedingly fine streams, solidifies as it meets the air. It cannot be shot out to any distance, but the animal usually draws it out by its hind legs, or attaches it to a spot, and moves away by walking or allowing itself to drop. It has some power of checking the output, and can stop at will at any point of its descent; but the sphincter muscles of the apertures are but weak, and by steady winding the writer has reeled out a hundred yards of the silk, the flow of which was only then interrupted by the spider rubbing its spinnerets together and breaking the thread.
There is, of course, no true spinning or interweaving of threads in the process, but parallel silken lines are produced, varying in number according to the special purpose for which they are designed, and sometimes adhering more or less to one another on account of their viscidity and closeness.
344The silk is utilised in many ways, serving for the construction of snares, nests, and cocoons, as well as for enwrapping the captured prey, and for anchoring the spider to a spot to which it may wish to return.
Spiders may be roughly distinguished as sedentary or vagabond, the former constructing snares, and the latter chasing their prey in the open. We will first consider the various forms of snare, beginning with that characteristic of the Epeiridae.
The Circular Snare.—This familiar object, sometimes spoken of as the orb-web or wheel-web, is always the work of some spider of the Family Epeiridae.
The accuracy and regularity of form exhibited by these snares has caused their architects to be sometimes called the geometric spiders. The ingenuity displayed by them has always excited the admiration of the naturalist, and this is increased on closer observation, for the snares are in reality even more complex than they appear at first sight.
The first care of the spider is to lay down the foundation threads which are to form the boundary lines of its net. If the animal can reach the necessary points of attachment by walking along intervening surfaces the matter is comparatively simple. The spinnerets are separated and rubbed against one of the points selected, and the spider walks away, trailing behind it a thread which it keeps free from neighbouring objects by the action of one of its hind legs. On reaching another desirable point of attachment the line is made taut and fixed by again rubbing the spinnerets against it. By a repetition of this proceeding a framework is presently constructed, within which the wheel or orb will ultimately be formed.
The process of fixing and drawing out a line can be conveniently watched in the case of a Spider imprisoned in a glass vessel, and it will be seen, by the aid of a lens, that a large number of very fine lines starting from the point of attachment seem to merge into a single line as the Spider moves away. This has given rise to the prevalent and very natural idea that the ordinary spider’s line is formed or “woven” of many strands. This, however, is not the case,[272] for the fine attachment-lines are not continued into the main thread, but only serve to anchor it to the starting-point.
345As has been said, the spider can throw into play a varying number of spinning tubes at will, and in point of fact those used in laying down these foundation-lines are either two or four in number. The spider, however, often finds it necessary to strengthen such a line by going over it afresh.
Every one must have noticed that orb-webs frequently bridge over gulfs that are clearly quite impassable to the spider in the ordinary way. They often span streams—and Epeirid spiders cannot swim—or they are stretched between objects unattainable from each other on foot except by a very long and roundabout journey. When this is the case, the animal has had recourse to the aid of the wind. A spider of this family placed on a stick standing upright in a vessel of water is helpless to escape if the experiment be tried in a room free from draughts. With air-currents to aid it, silken streamers will at length find their way across the water and become accidentally entangled in some neighbouring object. When this has happened, the spider hauls the new line taut, and tests its strength by gently pulling at it, and if the result is satisfactory, it proceeds to walk across, hand-over-hand, in an inverted position, carrying with it a second line to strengthen the first. This is exactly what happens in nature when a snare is constructed across chasms otherwise impassable, and it may be imagined that the spider regards as very valuable landed property the foundation lines of such a web, for, if destroyed, the direction or absence of the wind might prevent their renewal for days. They are accordingly made strong by repeated journeys, and are used as the framework of successive snares, till accident at length destroys them.
A single line which finds anchorage in this way is sufficient for the purposes of the spider. It has only to cross over to the new object, attach a thread to some other point of it, and carry it back across the bridge to fix it at a convenient spot on the surface which formed the base of its operations. Between two such bridge-lines the circular snare is constructed in a manner to be presently described. Sometimes the tentative threads emitted by the spider travel far before finding attachment. In the case of the English Epeira diademata the writer has measured bridge-lines of eleven feet in length; and with the great Orb-weavers of tropical countries they frequently span streams several yards in width.
346Two stout bridge-lines thus constructed will form the upper and lower boundaries of the net. The lateral limits are easily formed by cross lines between them at a convenient distance apart. The spider chooses a point, say, on the upper bridge-line, fixes its thread there, and carries it round to the lower line, where it is hauled taut and firmly attached. Two such cross lines give, with the bridge-lines, an irregular four-sided figure within which to stretch the snare, and now the work is perfectly straightforward, and can proceed without interruption.
Attention is first paid to the radii of the circular web. The first radii are formed by drawing cross lines within the framework in the same manner as before, but the spider carefully attaches these where they intersect by a small flossy mass of silk, and this central point or hub becomes the basis of its subsequent operations. It is a simple matter to add new radial lines by walking from the centre along one of those already formed and fixing the thread to some new point of the circumference. They are not laid down in any invariable order, but with a kind of alternation which has the general effect of keeping the strain on every side fairly equal. Almost every time the spider reaches the centre it slowly revolves, uniting the radii afresh at their point of junction, and increasing the strength and complexity of the hub. It also occasionally digresses so far as to stretch the whole structure by bracing the framework at additional points, so that it loses its four-sided form and becomes polygonal. We have now a number of spokes connecting a central hub with an irregular circumference.
The hub is next surrounded by what Dr. M‘Cook calls a “notched zone,” consisting of a few turns of spiral thread which serve to bind more firmly the spokes of the wheel. The most important part of the work is still to be performed. The lines hitherto laid down are perfectly dry and free from viscidity, so that an entangled insect would easily be able to free itself. A viscid spiral line remains to be spun, and the snare will be complete. The precise method of laying this down will vary somewhat according to the species, but, to refer again to the large Garden-spider, the proceeding is as follows:—Commencing at a point somewhat outside the notched zone, the creature rapidly works in a spiral thread of ordinary silk with the successive turns rather far apart. This forms a kind of scaffolding, by clinging 347to which the spider can put in the viscid spiral, which it commences at the circumference.
Its action now becomes exceedingly careful and deliberate, though by no means slow, and so great is its absorption in the work that it may be observed quite closely with a hand-lens without fear of interrupting it. The proceeding consists in drawing out from its spinnerets with one (or both) of its hind legs successive lengths of a highly elastic line, which it stretches just at the moment of fixing it to a radius, and then lets go with a snap. There is no hesitation or pause for consideration, but there is a peculiar deliberateness in drawing out each length of the thread which, together with stretching and sudden release, require explanation. Now, it has already been mentioned that the framework and radii of the snare are not at all moist or adhesive. This, however, is not the case with the spiral, upon which the spider chiefly relies in capturing its prey. A close examination of it—even with the naked eye—will show it to be beaded over with little viscid globules which, under a low magnifying power, are seen to be arranged with remarkable regularity.

Fig. 190.—A, B, C, D, Stages in the formation of the viscid globules of the web.
A very convenient method of investigation is to carry off a newly-constructed web—or, better still, one not quite finished—on a piece of plate glass, to which it will adhere by reason of the viscid spiral, and on which it may be examined at leisure. Immersion in a staining fluid will colour the viscid spiral, and show its structure in a striking manner. It will appear to consist of a thread strung with beads of two sizes, occurring with pretty uniform alternation, though two of the larger beads are often separated by two or more of the smaller.
Until recently it was supposed that the deposition of these beads upon the spiral line was a subsequent operation, and, in view 348of their vast numbers and regularity, the circumstance naturally excited great wonder and admiration. Blackwall[273] estimated that, in a fourteen-inch net of Epeira cornuta, there were at least 120,000 viscid globules, and yet he found that its construction occupied only about forty minutes! The feat, from his point of view, must be allowed to be rather startling.
As a matter of fact, the thread, on being slowly drawn out, is uniformly coated with viscid matter which afterwards arranges itself into beads, the change being assisted by the sudden liberation of the stretched line.
Boys[274] has shown their formation to be quite mechanical, and has obtained an exact imitation of them by smearing with oil a fine thread ingeniously drawn out from molten quartz. The oil arranged itself into globules exactly resembling the viscid “beads” on the spider’s line. If the web be carried bodily away on a sheet of glass, as above described, while the spider is engaged upon the spiral line, the experimenter will have permanent evidence of the manner in which the globules are formed. The last part of the line will be quite free from them, but uniformly viscid. Tracing it backwards, however, the beads are soon found, at first irregularly, but soon with their usual uniformity. The thread which the spider has thus “limed” for the capture of prey is really two-stranded—the strands not being twisted, but lying side by side, and glued together by their viscid envelope.
The snare is now practically complete, and the proprietor takes up her position either in the centre thereof, or in some retreat close at hand, and connected with the hub by special lines diverging somewhat from the plane of the web. Notwithstanding the possession of eight eyes—which, in sedentary spiders, are by no means sharp-sighted—it is mainly by the sense of touch that the spider presently becomes aware that an insect is struggling in the net. She immediately rushes to the spot, and suits her action to the emergency.
If the intruder is small, it is at once seized, enveloped in a band of silken threads drawn out from the spinnerets, and carried off to the retreat, to be feasted on at leisure. If it seems formidable it is approached carefully—especially if armed with a sting—and silk is deftly thrown over it from a safe distance till it is thoroughly entangled, and can be seized in safety by the 349venomous jaws of its captor. Sometimes the insect is so powerful, or the spider so sated with food, that the latter hastens to set free the intruder by biting away the threads which entangle it before much havoc has been wrought with the net.
The viscid matter on the spiral line dries up after some hours, so that, even if the web has not been destroyed by insects and stress of weather, this portion of it must be frequently renewed. Commencing a new web is, as has been seen, a troublesome matter, and it will readily be understood that the spider prefers, where practicable, to patch up the old one. This is done by biting away torn and ragged portions and inserting new lines in their place.
The part played by the various spinning glands in the construction of the orb-web may be briefly stated.[275] The ampullaceal glands furnish the silk for the foundation lines and radii. The spiral has a double ground-line proceeding from the middle spinnerets, but it is not quite certain whether it proceeds from the ampullaceal or the tubuliform glands. The chief function of the latter, in the female, is to furnish silk for the egg-cocoon. The viscid globules are the products of the aggregate glands. The aciniform and piriform glands provide the multitudinous threads by which the spider anchors its various lines and enwraps its prey.
Some Orb-weavers always decorate their snares with patches or tufts of flossy silk. In the snare of the North American Argiope cophinaria the hub is sheeted, and from it extends downwards a zigzag ribbon of silk stretched between two consecutive radii. Vinson[276] discovered a remarkable use for similar zigzag bands in the web of the Mauritian spider, Epeira mauritia. It furnished a reserve supply of silk for enveloping partly entangled insects whose struggles were too vigorous to succumb to the rather feeble threads which the spider was able to emit at the moment of capture. The spider was able to overcome a grasshopper much more powerful than itself by dexterously throwing over it with one of its hind legs a portion of the ribbon of silk which it had thus stored up for emergencies.

Fig. 191.—A, Snare of Hyptiotes cavatus; B, enlarged view of the Spider, showing the “slack” of the hauled-in line. (After Emerton.)
Many orb-webs are defective, a sector of the circle being uniformly omitted in the structure. The genus Hyptiotes does 350not belong to the Epeiridae but to the cribellate Uloboridae, but its defective orb-web is so curious that it deserves a special mention. A single foundation-line is laid down, and from it four radii are drawn and are connected with cross lines, the snare constituting about one-sixth of a circle. From the centre of the incomplete circle a thread proceeds to some more or less distant object, and on this the spider takes up its position, inverted, and hauls in the line till the snare is taut. When the trembling of the line shows the spider that an insect has struck the net, it lets go with its fore-legs, and the web, springing back to its normal position, entangles the intruder more thoroughly by its vibrations. When large insects are in question the spider has been observed to “spring” the net several times in succession. H. cavatus is common in the pine woods of Pennsylvania, but the only English species, H. paradoxus, is extremely rare.
A remarkable spider has been discovered in Texas by M‘Cook, which, after building a horizontal orb-web, converts it subsequently into a dome (Fig. 192) of exceedingly perfect form. It is named Epeira basilica, and has been the object of careful study by Dr. Marx, who observed the whole process of web-construction. Threads are attached at various points on the upper surface of the horizontal wheel, the central portion of which is gradually pulled up until the height of the dome is nearly equal to the diameter of its base. But the snare of this spider does not consist of the dome alone. A sheet of irregular lines is stretched below, while above there is a maze of threads in the form of a pyramid. Several other Orb-weavers, as, for instance, E. labyrinthea and E. triaranea, supplement their typical webs by an 351irregular structure of silk, and thus form connecting links, as regards habit, between the group of which we have been speaking and the Theridiidae or Line-weavers, which may now briefly be dealt with.

Fig. 192.—Snare of Epeira basilica. (After M‘Cook.)
The Irregular Snare.—The great majority of British Spiders belong to the family of the Theridiidae, or Line-weavers. Some of these are among the handsomest of our native species, and are in other respects highly interesting, but their snares lack the definiteness of structure exhibited by the orb-web, and little need be said about them.
For the most part they consist of fine irregular lines running in all directions between the twigs of bushes or among the stems of grass and herbage. One large and important genus, Linyphia, always constructs a horizontal sheet of irregular threads with a maze of silk above it. Such snares may be seen in myriads in the wayside hedges during the summer, and they are especially notable objects when heavily laden with dew. Insects impeded in their flight by the maze of threads drop into the underlying sheet, and are soon completely entangled. The spider usually runs beneath the sheet in an inverted position.
The sheet or hammock of silk is absent in the case of most of the other genera of this family, their snares being innocent of any definite method in their structure. They are frequently quite contiguous, and it is no uncommon thing to find a holly bush completely covered with a continuous network of threads, the work of a whole colony of the pretty little spider Theridion sisyphium.
As might be imagined from the simplicity or absence of design in the structure of the net, there appears to be very little 352complexity in the nature of the silk used. It is interesting, however, to find that viscid globules, not unlike those which stud the “spiral line” of the Epeiridae, are sometimes present in the snares of the Line-weavers,[277] and in these, too, aggregate glands are present. There is a large spider of this family, Theridion tepidariorum, which may be found to a certainty in almost any hothouse in this country. In its snare, which is of the ordinary irregular type, F. Pickard-Cambridge has observed little patches of flocculent silk, calculated to render more certain the entanglement of prey, and he has further described a curious comb-like structure on the hind leg of the animal which is probably used in the production of this phenomenon. It is by no means unlikely that a more careful study of these apparently simple snares will lead to the discovery of further complexity of structure.

Fig. 193.—Snare of Uloborus sp., some of the lines being thickened with threads from the cribellum. (After M‘Cook.)
Uloborus, a cribellate genus which has an Epeirid-like, orbicular snare, decorates some of the lines with the produce of the cribellate glands, but viscid globules are absent.
Sheet-Webs.—The webs which are such familiar—and, by association, unpleasant—objects in unused rooms and outhouses are usually the work of spiders belonging to the Agelenidae and the Dictynidae. To the first belongs the common House-spider, Tegenaria civilis, and its larger congener, T. parietina. These spiders are not attractive in appearance, and the last-named species especially, with the four-inch span of its outstretched legs, is a formidable object, and a terror to domestic servants. An obscure tradition connecting it with Cardinal Wolsey and Hampton Court has caused it to be known as the Cardinal Spider. An out-door example of the Agelenidae is the very abundant Agelena labyrinthica, whose sheet-web, with its tubular retreat, is to be sought on the banks of ditches, or in the hedges of our country lanes.
353The snares of these spiders are exceedingly closely woven of very fine silk, and take a long time to complete. The process of their construction may be watched by keeping an Agelena labyrinthica confined in a box with a glass front, and the web, kept free from dust, is a beautiful object, as its fine texture gradually becomes visible as a delicate transparent film which develops by imperceptible stages into an opaque white sheet. The excessive fineness of the silk makes it difficult at first to see what is taking place. The animal is seen to be busily moving about, but the result of its labours only gradually becomes visible. A few delicate foundation-lines are first stretched across the compartment in which it is confined, and upon these the spider walks to and fro incessantly with a serpentine motion, and by and by a muslin-like floor of silk comes into view.
An examination of the spinnerets throws some light upon the operation. The posterior pair are very long and mobile, and the hair-like spinning-tubes are distributed on their under surface. The cephalothorax and abdomen are far more rigidly connected in Agelena than in the Orb-weavers, but its length of leg and the length and mobility of its posterior spinnerets enable it to give a wide lateral sweep as it walks along, strewing fine silken threads upon the foundation-lines already laid down. Some hours elapse before even a moderately stout web is constructed, and for long afterwards the spider devotes odd moments of leisure in going over the ground again and strewing new silk upon the gradually thickening web. At one corner a silken tube of similar structure is formed, and in this the spider awaits the advent of any insect which may alight upon the sheet, when it immediately rushes forth and seizes it.
The webs of the Dictynidae are very similar in general appearance to those of the Agelenidae, consisting of a closely-woven sheet with a tubular nest. They are to be found, moreover, in similar situations, stretching across the angles of walls in cellars or outhouses, though some species prefer an out-door existence. Crannies in rock form convenient sites for such snares, but the family is not without its representatives in still more open situations. The web, though so similar to that of Agelena, is, however, constructed in a different manner. In the Dictynidae neither the legs nor the spinnerets are unusually long, and they do not strew the foundation-lines by a swinging motion 354of the body, but the operation is effected by a special apparatus. These spiders are cribellate, and in front of the six ordinary spinnerets there are a pair of perforated plates connected with a large number of additional minute spinning glands (see Fig. 182, p. 326). In conjunction with this, the female possesses on the last joint but one of each hind leg a curious comb-like arrangement of spines, the “calamistrum.” The animal constructs a sort of skeleton web by means of its ordinary spinnerets, and when this is completed it combs out silk from the cribellum by means of the calamistrum, using each hind leg alternately, and distributes it with a curling motion upon the scaffolding prepared for it, a nearly opaque web being the result. The silk from the cribellum is of an adhesive nature, and renders escape from the web very difficult.
Spiders’ Nests and Retreats.—All Spiders construct some description of nest, and often display great ingenuity in building them. Perhaps none are more curious than those of the burrowing Aviculariidae, a family which includes the interesting “Trap-door Spiders.” They are nocturnal in their habits, about which, consequently, little is known, but their nests have been carefully studied, especially by Moggridge, who found them in considerable abundance in various districts of the Riviera.
The jaws of these spiders are especially adapted for digging, and with them a hole is excavated in the ground to the depth of several inches, and wide enough to allow the animal to turn. This is carefully lined with silk which the spider throws against the sides from its long and upturned posterior spinnerets. But the chef d’œuvre of the whole structure is a lid or door which protects the entrance to the tube. There are two types of door which find favour with different species—the wafer and the cork type, as Moggridge has named them. The former consists of a thin circular or oval sheet of silk which flaps down loosely over the tube-entrance, with which it is connected by a hinge-like attachment. A trap-door of the cork type is a more complicated structure, being of considerable thickness and having a bevelled edge, so that it fits into the tube like a plug. Like the wafer door, it possesses a silken hinge.
To form the wafer door, the spider covers the entrance to the tube with a closely-woven layer of silk, which it afterwards bites away at the edge, except at the point where the hinge is to be. 355Doors of the cork type consist of alternate layers of silk and earth. After weaving a covering of silk, the creature brings earth in its jaws and lays it on the top, binding it down with a second layer of silk, and the process is repeated until the requisite thickness is attained.
The nests are exceedingly difficult to detect, as the spiders take the precaution of attaching leaves, moss, or small twigs to the outer surface of the doors. This does not appear to be the result of intelligence, but a mere instinctive habit; for if a door be removed and the surrounding earth denuded of moss, the spider will render the new door conspicuous by bringing moss from a distance, and thus making a green spot in the bare patch of earth.[278]
The cork doors fit with great exactness, and there is always to be found on their under surface a notch by which they are held down by the fore-legs of the spider against any attempt to open them from without.
Many nests with trap-doors of the wafer type are found to have a second and more solid door within the tube. This serves to shut off the lower part of the nest as a still more secure retreat. This second door opens downwards, and the Spider, getting beneath it, is effectually shielded from an enemy which may have mastered the secret of the outer barrier. The nests of some species present still further complications in the way of lateral branches from the main tube. In one case (Nemesia congener) the burrow becomes Y-shaped, and the second door hangs at the fork of the Y in such a manner as to connect the bottom chamber either with the entrance or with the branch, which does not reach the surface, but ends blindly.
Trap-door Spiders are greatly attached to their tubes, which they enlarge and repair at need. They begin burrowing very early in life, and their tiny tubes resemble in all respects those of their parents. Their habits are nocturnal, and little is known of them; an observation, however, on a species inhabiting the island of Tinos in the Grecian Archipelago (Cteniza ariana), by Erber,[279] must not be omitted. This spider leaves its tube at night and spins a web near at hand and close to the ground. It carries captured insects into its tube, and in the morning 356clears away the net, adding the material of it, M. Erber believes, to the trap-door.
No true trap-door Spider has as yet been found in this country, but the allied Atypidae are represented by at least one species, Atypus affinis, which has been discovered in colonies in some localities in the south of England, notably near Ventnor in the Isle of Wight, and on Bloxworth Heath in Dorsetshire. This spider, like its continental cousins, excavates a hole in the earth, generally near the edge of a heathery bank, and lines it with a tube of silk of such firm texture that it may be removed intact from the earth in which it is embedded. The silken tube projects some two inches above the ground, either erect among the roots of the heather, or lying loosely upon the surface. Its extremity is always found to be closed, whether from its own elasticity or by the deliberate act of the proprietor is uncertain, and it seems probable that the animal spends almost the whole of its existence in the tube. Simon believes that it feeds almost entirely upon earth-worms which burrow into its vicinity, and which it, therefore, need not leave its nest to catch; but the remains of beetles and earwigs have been found in the tubes at Ventnor.

Fig. 194.—Funnel of Cyrtauchenius elongatus. (After M‘Cook.)
This description of nest seems common to all species of the genus Atypus. The American “Purse-web Spider,” A. abboti, burrows at the foot of a tree, against the trunk of which it rears the projecting portion of its silken tube. At the bottom of the nest the cavity is enlarged, and blind processes project in different directions.
Another burrowing spider, Cyrtauchenius elongatus, surmounts its silk-lined burrow by a funnel-shaped structure of pure white silk, about three inches in height and two or three inches in width. There is no attempt at concealment, and the white funnels are conspicuous among the thin grass, presenting the appearance of fungi.
The burrowing habit is also common to the Wolf-spiders or 357Lycosidae, but beyond a very slight lining of silk there is usually little spinning work about their nests. Occasionally there is a certain amount of superstructure in the shape of a silken funnel (Lycosa tigrina, M‘Cook), or of an agglomeration of twigs and pebbles, as in the case of the “Turret-spider” (Lycosa arenicola, Scudder).

Fig. 195.—Turret of Lycosa carolinensis. (After M‘Cook.)
A colony of our handsome species, Lycosa picta, is an interesting sight to watch. Their favourite habitat is a sandy soil, variegated with many-coloured patches of moss and lichen, among which their own markings are calculated to render them inconspicuous. The observer, by lying perfectly still, may see them silently stealing forth from their burrows in the bright sunshine, and hunting diligently in the neighbourhood, ready to dart back on the faintest alarm, or if the sun should be temporarily obscured by a passing cloud. So closely do they resemble their surroundings, that it is only when in motion that they can readily be detected. It is very curious to see them popping out their heads to ascertain that the coast is clear before venturing forth, and the utter silence of their operations adds to the eeriness of the effect. The tubes of these spiders, though without a trap-door, and only slightly lined with silk, are Y-shaped like those of Nemesia congener, the main tunnel giving off a blind branch about half-way down.
The nest of the Water-spider, Argyroneta aquatica, must not be passed over without mention. This spider, though strictly an air-breathing animal, spends almost the whole of its existence beneath the water. That it can live in such a medium is due to the fact that the long hairs which clothe its abdomen retain a bubble of air as it swims beneath the water, so that it carries with it its own atmosphere. The air-bubble which invests its body gives it a strong resemblance to a globule of quicksilver, and renders it a pretty object in an aquarium as it swims about in search of food or in prosecution of its spinning operations.
Of these the most interesting is the building of its nest. 358Working upon a water plant some distance below the surface, it forms a silken dome of closely-woven threads, which it next proceeds to fill with air. To do this the spider rises in the water, raises its abdomen above the surface, and jerks it down again quickly, so as to carry with it a bubble of air which it helps to retain with its hind legs. With this it swims back to its tent, into which it allows the imprisoned air-globules to escape. By degrees the dome or bell is filled, and the creature has a dry and snug retreat beneath the water. In this it passes the winter in a torpid condition. The young of this species appear to be fond of utilising the empty shells of water-snails, which they float by filling them with air, and thus save themselves the trouble of nest-construction.

Fig. 196.—Egg-cocoons. A, Epeira diademata, nat. size. B, Theridion pallens × 4, attached to a leaf. C, Agroeca brunnea, nat. size, attached to a weed, and not yet coated with mud. D, Ero furcata × 4, attached to a log.
Cocoon.—The last important spinning operation which remains to be described is the building of the so-called cocoon. This must be distinguished from the cocoon of insects, which is a protective covering of silk within which the larva assumes the pupa form. In the case of the Spider, the term is applied to the structure which serves to protect and conceal the eggs. It is often of considerable complexity, and is highly characteristic of the particular species which constructs it.
All egg-bags are commenced in very much the same way. A small sheet of silk is woven, and against this, sometimes upon the upper and sometimes on the under surface, the eggs are deposited, and then covered in with a second silken layer. The compact silk-covered ball of eggs is then, in many cases, enclosed in a small compartment which the spider builds with infinite care and unfailing uniformity, after the pattern peculiar to its kind. A considerable number of the Orb-weavers are content with a 359simple silken case closely investing the eggs, and by its thickness and the non-conducting quality of the material, sufficient protection is afforded against inclement weather.
The egg-bag of the large Garden-spider (E. diademata) may be recognised by its great size and its yellow colour, which is deepened by the still more yellow tint of the eggs within. Those of Zilla x-notata and of many other English Epeirids are of similar structure, but of white silk. The mother generally avails herself of some natural shelter, hiding her cocoon beneath loose bark, in the crannies of masonry, or under the copings of walls.
Many species, on the contrary, boldly expose their cocoons in their snares, sometimes as many as fourteen being constructed in succession and strung in a chain. The American species Epeira caudata and E. bifurca are good examples of this habit, stringing a chain of characteristic cocoons upon the line connecting the retreat with the web.
The sedentary Theridiid spiders usually suspend their cocoons in the neighbourhood of their irregular snares. The green cocoon of Theridion sisyphium is generally more or less concealed by an accumulation of débris. The minute species T. pallens constructs a cocoon of peculiar shape on the under surface of a leaf (Fig. 196, B). It is a conical structure of white silk, considerably larger than the spider itself, attached at its broad end,[280] and having several curious lateral projections near the middle.
Among the Lycosidae or “Wolf-spiders” the prevailing habit of the mother is to carry the egg-bag attached beneath her abdomen upon all her hunting excursions. It is spheroidal in shape, made up of an upper and a lower half, with a seam-like junction at the equator, so to speak. The lower half is first woven, and the eggs are deposited within it. The upper hemisphere is then spun, and the edges gathered in and finished off, the seam or suture being always discernible. The bag is now attached by silken threads to the spinnerets, and bumps merrily over the ground as the animal hurries along in search of prey. If deprived of it she evinces the greatest distress, and frequently will not try to escape without it.
Attempts to utilise Spider Silk.—It is long since the web 360of the House-spider, taken internally, was considered a specific for the ague, though its value as a styptic has been recognised in quite recent times. It is, however, with other uses of Spider silk that we are here concerned.
Spider silk has been extensively used in the micrometer eyepieces of telescopes where very fine intersecting lines are required. For this purpose the radial or scaffolding lines of the circular snare were selected, the spiral being unsuited on account of its row of viscid beads. Professor C. V. Boys has, however, discovered in his quartz fibres a material better adapted for this purpose.
Several attempts have been made to weave the silk of Spiders as a substitute for that of the silk-worm. Web silk is, of course, far too fine to furnish a durable material, but the cocoons are usually formed of coarser silk, and it is with them that the experiment has been tried. About the beginning of the eighteenth century certain stockings and mittens made of Spider silk from the cocoons of Epeira diademata, by M. Bon of Languedoc, attracted so much attention that the Academy desired M. Réaumur to investigate the matter. His report was unfavourable to the commercial utility of Spider silk. The cocoon threads, though eighteen times stronger than those of the web, were but one-fifth of the strength of those obtained from the silk-worm, and the lustre was inferior. A still more fatal objection, however, was founded upon the cannibalistic habits of the spider, and the difficulty of furnishing it with acceptable food.
M. Vinson has recorded that some of the spiders of Madagascar, especially Epeira madagascarensis, are far better adapted than any of our English species to a commercial use. They furnish silk of a beautiful clear yellow colour; they are accustomed to live harmoniously together in families; and the range of climate in which they can thrive is very considerable. The Creole ladies of this island, under the administration of General Decaen, wove a magnificent pair of gloves from spider silk, with their own hands, for presentation to the French Empress.
Poison of Spiders.—All spiders possess poison-glands, which have their openings on the fangs of the chelicerae. The action of the chelicera in striking does not express the venom, but the poison-bag itself is covered with a muscular coat by which the contained fluid is expelled. It is highly probable, 361therefore, that the venom is under the control of the animal’s will, and is economised when the simple wound is sufficient for the purpose—a supposition which may partially explain the very divergent opinions held with regard to the effect of the spider’s bite. The reputation of the “Tarantula” Spider is well known, but what particular species, if any, was intended by the name is quite uncertain. The name is derived from the town Tarentum, and was certainly applied to a Lycosid spider. Probably the common south European species, Lycosa narbonensis, has as good a claim to the honour as any. The confusion has been increased by extending the name to spiders of quite a different family. Eurypelma hentzii, one of the Aviculariidae, is commonly known as the Tarantula in America.
The superstition of the tarantula dance is well known. The bite of the spider was supposed to induce a species of madness which found its expression—and its cure—in frantic and extravagant contortions of the body. If the dance was not sufficiently frenzied, death ensued. In the case of survivors, the symptoms were said to recur on the anniversary of the bite. Particular descriptions of music were supposed to incite the patient to the excessive exertion necessary for his relief; hence the “Tarantella.”
In the Middle Ages epidemics of “tarantism” were of frequent occurrence, and spread with alarming rapidity. They were seizures of an hysterical character, analogous to the ancient Bacchic dances, and quite unconnected with the venom of the spider from which they took their name. The condition of exaltation and frenzy was contagious, and would run through whole districts, with its subsequent relapse to a state of utter prostration and exhaustion. The evil reputation of the Tarantula appears to have exceedingly little basis in fact.
Baglivi relates how the country people capture the Tarantula by imitating the buzzing of an insect at the mouth of its hole. “Quo audito, ferox exit Tarentula ut muscas, quorum murmur esse putat, captet; captatur tamen a rustico insidiatore.”
Fabre[281] acted the part of the “insidious rustic” with slight success; but by other stratagems he enticed the creatures from their holes, and made some interesting observations upon the effects of their bite. He found that bees and wasps were instantaneously killed by them. This immediately fatal effect he 362found to be due to the fact that the spider invariably struck the insect in a particular spot, at the junction of the head with the thorax. Bees must often wander into Tarantula’s holes, and a prolonged contest, though it might end in the death of the insect, would be certain also to result fatally for the spider. It has, therefore, acquired the habit of striking its foe in the one spot which causes instant death. When Fabre presented a bee to a Tarantula in such a manner that it was bitten in some other region, the insect survived several hours.
A young sparrow, just ready to leave the nest, was bitten in the leg. The wound became inflamed, and the limb appeared to be paralysed, but the victim did not at first suffer in general health, and fed heartily; death resulted, however, on the third day. A mole died in thirty-six hours after the bite.
From these experiments, Fabre came to the conclusion that the venom of the Tarantula was at all events too powerful to be entirely negligible by man.

Fig. 197.—Latrodectus mactans, ♂, natural size.
Trifling causes may have a fatal effect upon a man in ill health, and it is quite possible that death has sometimes resulted from the Tarantula’s bite. Its effect upon a healthy subject, however, is certainly not serious. Goldsmith, in his Animated Nature, entirely discredits the current stories about this animal, saying that the Italian peasants impose upon credulous travellers by allowing themselves, for money, to be bitten by the Tarantula, and then feigning all the symptoms which are traditionally supposed to ensue.
There is a genus of the Theridiidae, by name Latrodectus, whose poisonous reputation almost rivals that of the Tarantula. It is remarkable, moreover, that it is regarded as particularly dangerous in such widely separated portions of the world as Madagascar, New Zealand, Algeria, the West Indies, and North America. These spiders, strangely enough, are by no means particularly large or formidable in appearance.
There are two species in Madagascar, known to the natives by the names of Mena-vodi and Vancoho. Vinson[282] describes the 363terror which is locally inspired by the first-named species, whose bite is believed to be fatal unless measures are promptly taken to counteract the poison. They sometimes cauterise the wound, but the usual treatment consists in inducing profuse perspiration—a method of cure which recalls the Tarantula dance of Southern Europe. Flacourt[283] mentions the Vancoho as the most dangerous animal of Madagascar, and more formidable than the scorpion. He relates cases among his own negroes where the bite was followed by a condition of syncope which lasted two days.
A New Zealand species is known by the natives as the Katipo. It is of about the size of a pea, and almost black in colour. Mr. Meek of Waiwera gives a most circumstantial account of the effect of its bite upon his son.[284] During the four days which followed the bite he suffered excruciating pain, which spread from his leg to the spine, arms, and chest, and he lost twelve pounds in weight. Relief was obtained by frequent doses of brandy and the use of a liniment.
The natives of New Zealand have a great horror of this spider, but hold the curious belief that its death will ensure the cure of any one it may have bitten. If unable to find it, they will burn the house down rather than allow it to escape. Their dread, however, is confined to a variety which lives among the sedge of the sea-beach, and they carefully avoid sleeping in such places.
Two of the best authenticated cases of serious results ensuing from the bite of a spider of this genus come from North Carolina.[285]
A farm labourer in the employ of Mr. John Dick of Greensborough was bitten by Latrodectus mactans about half-past eight in the morning, and died between ten and eleven o’clock at night. Small pimples were raised in the neighbourhood of the bite, but no puncture was discernible. Intermittent pains and spasms ended in a comatose condition from which he did not rally. The man appeared previously to be in perfect health.
Another man on Mr. Dick’s farm was bitten by the same species of spider. He resumed work, but a spasm of pain caused him to mount his horse and endeavour to ride home, but he fell off, and lay in a state of unconsciousness. He was found in this condition by a fellow-workman, and taken home. Large quantities 364of whisky were administered without any intoxicating effect, and this afforded some relief from the constantly-recurring spasms. The paroxysms continued for three weeks, and two months elapsed before he was able to resume work. On the ankle where he was bitten pimples appeared as in the previous case, and these broke out again, long after the occurrence, whenever he became overheated in his work.
These accounts are sufficiently circumstantial and well authenticated, but the fact of the actual bite depends upon the statement of the victims alone, and they may possibly have mistaken the cause of their trouble.
Southern Europe possesses a congener of this spider in Latrodectus 13–guttatus, the well-known “Malmignatte,” which is also considered extremely poisonous. The Royal Academy of Medicine and Surgery at Barcelona appointed Dr. Graells, in 1833, to inquire into the effects of the bite of this spider, cases of which had become exceedingly frequent. He found a curious correspondence between the frequency of these cases and the advent of migratory locusts, which the spider successfully attacked. In his report[286] he details the symptoms in certain unquestionably authentic cases. There was a double puncture, surrounded by red circles, the region of the wound afterwards swelling greatly. The pain and swelling extended over the whole limb, and often to the body, and convulsions occurred, followed by great prostration and collapse. All the patients eventually recovered, their cure being heralded by profuse perspiration.
It must be mentioned, however, that the eminent Arachnologist M. Lucas states that he has several times allowed himself to be bitten by this identical spider without any ill effects.
The testimony is thus conflicting in this case also. It is impossible, however, to believe that there is no basis in fact for the poisonous reputation of a comparatively insignificant-looking spider in so many widely separated parts of the world, supported as it is by certain well-substantiated cases. The variable effects of its bite may find a partial explanation in a variation in the strength of its venom at different seasons, and it has already been mentioned that the injection of poison into its victim is a voluntary act, and does not necessarily accompany its bite. Among the 365species regarded as especially venomous must be mentioned Phidippus morsitans, one of the larger of the Attidae.
It is exceedingly likely that the bite of the large tropical Aviculariidae is really formidable. They appear, however, more anxious to escape than to show fight, and we possess little reliable information with regard to them. Doleschall shut up small birds with two West Indian species, and death followed their bite almost immediately. Ten days’ starvation appeared to weaken the venom, for a bird bitten by a spider fasting for that period recovered after an indisposition of six hours.
Most Arachnologists have recorded experiments with regard to the venom of the commoner European species, with equally conflicting results. Blackwall came to the conclusion that loss of blood, and not poison, caused the death of spider-bitten insects. He could not himself distinguish a spider bite from the prick of a needle inflicted upon his hand at the same time. Bees, wasps, and grasshoppers survived the bite about as long as other insects of the same species outlived a needle-prick in the same part of the body. Walckenaer’s experience was of the same nature. Bertkau, however, when bitten in the hand, felt clear indications of an irritant poison in the wound. The hairs of some of the large hairy species of the Aviculariidae possess poisonous properties. They are readily parted with, and when the animal is touched by the hand considerable irritation is set up.
Fertility of Spiders.—Spiders vary greatly in the average number of eggs laid by different species, and within the limits of each species there is a very considerable variation in fertility. As a rule it appears that the large and vigorous spiders are more prolific than the smaller and weaker members of the order. Were all the facts before us, however, we should no doubt find that the number of eggs laid bore a direct proportion, not to the size of the species, but to the dangers to which the young of that species are exposed. Where the total numerical strength of a species is fairly stationary, such a proportion must of course exist. Some species, no doubt, are tending to become extinct, while others are increasing in numerical importance. As a general rule, however, it is safe to infer that, if a species is especially prolific, special dangers attend the rearing of the young. The largest of North American Epeirids, Argiope cophinaria,[287] 366constructs a cocoon containing, on an average, 1150 eggs. As many as 2200 have been counted in exceptional cases. Even this number is exceeded in the case of some of the great Aviculariidae. Theraphosa leblondi deposits as many as 3000 eggs. The large European Epeirids, E. quadrata and E. diademata, lay about 600 eggs, those of Lycosa narbonensis reaching about the same number. Those American spiders which have been described as stringing up a series of cocoons in their webs usually attain about the same aggregate, the eggs being less numerous in each cocoon.
These are examples of fairly large and fertile spiders. In the case of other species the number of eggs laid is exceedingly small. Ero furcata makes a single cocoon containing six eggs. Synageles picata, an ant-like Attid, lays only three. Oonops pulcher constructs several cocoons, but each contains only two eggs. The eggs of Cave-spiders, and such as live in dark and damp places, are generally few in number. Anthrobia mammouthia, for example, an inhabitant of the great American caves, deposits only from two to five eggs.
Our knowledge of the special perils which beset particular species is so incomplete that we are often at a loss for the reason of this great inequality in fertility. For instance, how does Synageles picata maintain its numerical strength by laying only three eggs, when, as M‘Cook points out, its resemblance to the ant, though advantageous to the adult spider, affords no protection to the egg? Our knowledge must be greatly extended before we are able to account for particular cases. Many influences hostile to spiders as a group are, however, well known, and we may here enumerate them.
Natural Enemies.—The precautions taken by the mother in constructing the cocoon render the inclemency of the weather very much less destructive to the eggs than to the newly-hatched young. Nevertheless, among spiders inhabiting swampy regions great havoc is wrought by the occasional wholesale swamping of the cocoons by floods. Professor Wilder considers the great fertility of Nephila plumipes necessary to counterbalance the immense destruction worked by the heavy rains upon their cocoons, which are washed in great numbers from the trees, to the leaves of which they are attached. But such exposed situations are avoided by many species, and their eggs, enclosed in 367their silken envelope, are well protected against the severities of the weather.
A more universal enemy to the egg is found in Ichneumon flies. On examining the cocoons of almost any species of spider, a large proportion are almost certain to be found to contain Ichneumon larvae. Mr. F. Smith, in the Transactions of the Entomological Society for 1860, describes two species, Hemeteles fasciatus and H. formosus, which are parasitic on the eggs of Agelena brunnea. They are figured in Mr. Blackwall’s book on British Spiders. Pezomachus gracilis attacks the cocoons of many kinds of American spiders, appearing to have no special preference for any particular species, while Acoloides saitidis seems to pay special attention to the eggs of certain of the Jumping-spiders, and particularly of Saitis pulex.
The Ichneumons which thus regard the Spider’s eggs as convenient food for their own larvae are probably very numerous. Nor are they themselves always free from parasites. Occasionally the larvae of minute Hymenopterous insects are found to be parasitic upon the eggs of an Ichneumon which have been laid in a Spider’s cocoon.
It sometimes happens that the development of the young spiders has so far advanced at the time of the Ichneumon’s intrusion that the latter’s intention is frustrated, and its offspring, instead of devouring, are themselves devoured. Again, some few of the eggs in an infested cocoon occasionally escape the general destruction and reach the adult condition, but there can be no doubt that Ichneumons are largely instrumental in keeping down the numbers of most species of spiders. The perils which attend the Spider after leaving the cocoon are no less formidable, and much more numerous. The whole newly-hatched brood may be destroyed by a heavy rain-storm. If there is not a sufficient supply of food suitable to their feeble digestive powers they perish of inanition, or eat one another. This cannibalistic propensity is a considerable factor in the mortality among young spiders, and the adult animals frequently prey upon one another.
Argyrodes piraticum, in California, invades the webs of larger spiders of the family Epeiridae, which it seizes and devours. A. trigonum, common in the eastern states of North America, has the same habit.[288] Hentz found in Alabama a spider, which he named 368Mimetus interfector, of still more ferocious and piratical habits. Its special quarry is Theridion tepidariorum. Sometimes the Theridion overcomes the invader, and one case was observed in which a second Mimetus was devouring a Theridion beside the dead body of its predecessor, who had come off the worse in the combat.
The eggs of Theridion tepidariorum are also sometimes devoured by this spider, and a similar propensity has been observed in some English species, for Staveley[289] states that it is common to see certain spiders of the genus Clubiona feeding upon the eggs which have been laid by their neighbours. The larvae of some Hymenopterous insects are parasitic upon Spiders themselves, and not upon their eggs. Blackwall found this to be the case with the larvae of Polysphincta carbonaria, an Ichneumon which selects spiders of the genera Epeira and Linyphia on which to deposit its eggs.[290] The spider thus infested does not moult, and is soon destroyed by the parasite which it is unable to dislodge from its back. Menge, in his Preussische Spinnen, enumerates several cases of parasitism in which the larva, as soon as it has developed from the egg, enters the spider’s body, there to continue its growth. Spiders are also subject to the attack of a parasitic worm, Gordius (cf. vol. ii. p. 173).
Some of the most deadly foes of Spiders are the Solitary Wasps. There are many species of Pompilus (vol. vi. p. 101), which, having excavated holes in clay banks, store them with spiders or other creatures which they have paralysed by their sting. They then deposit an egg in the hole, and immediately seal up the orifice. This habit is found to characterise the solitary wasps of all parts of the world. Belt[291] relates the capture of a large Australian spider by a wasp. While dragging its victim along, it was much annoyed by the persistent presence of two minute flies, which it repeatedly left its prey to attempt to drive away. When the burrow was reached and the spider dragged into it, the two flies took up a position on either side of the entrance, doubtless with the intention of descending and laying their own eggs as soon as the wasp went away in search of a new victim. Fabre[292] gives an interesting account of one of the largest European Pompilidae, 369Calicurgus annulatus, which he observed dragging a “Tarentula” to a hole in a wall. Having with great difficulty introduced its burden into the cavity, the wasp deposited an egg, sealed up the orifice, and flew away. Fabre opened the cell and removed the spider, which, though completely paralysed, lived for seven weeks.
The same indefatigable observer describes the method adopted by the comparatively small Pompilus apicalis in attacking the formidable Wall-spider, Segestria perfida. The combatants are well matched, and the issue of the battle would be doubtful if the wasp did not have recourse to stratagem. Its whole energies are directed towards forcing the spider away from its web. At home, it is confident and dangerous; when once dislodged, it appears bewildered and demoralised. The wasp darts suddenly towards the spider and seizes it by a leg, with a rapid effort to jerk it forth, releasing its hold before the enemy has had time to retaliate. The spider, however, as well as being anchored by a thread from its spinnerets, is clinging to its web with its hind legs, and if the jerk is not sufficiently energetic, it hastily scrambles back and resumes its defensive position. Before renewing the attack the wasp gives the spider time to recover from the excitement of the first onset, seeking, meanwhile, the retreats of other victims. Returning, it succeeds, by a more skilful effort, in drawing the spider from its retreat and hurling it to the ground, where, terrified and helpless, it falls an easy prey. Should the insect bungle in its first attack and become entangled in the web, it would itself become the victim. Certain wasps thus appear to seek out particular species of spiders as food for their larvae. Others are less discriminate in their tastes. Again, some, as in the cases cited above, store their egg-nest with a single spider, while others collect many for the purpose.
The American “blue digger wasp” (Chlorion caeruleum) excavates its nest in the ground, and inserts a single large spider of any species.[293] Another wasp, of the genus Elis, selects the Wolf-spiders, and especially Lycosa tigrina, for the use of its larvae, while Priocnemus pomilius shows a preference for the Crab-spiders, or Thomisidae.
One of the most remarkable instances is that of Pepsis 370formosa, which preys upon the gigantic spider Eurypelma hentzii, wrongly styled in America the “tarantula,” but really belonging to an entirely different family, the Aviculariidae.
Fabre’s most interesting researches have established the fact that the instinct of the wasp leads it to sting the spider in a particular spot, so as to pierce the nerve-ganglion in the thorax. The precision with which this is effected is absolutely necessary for the purpose of the insect. If stung elsewhere, the spider is either incompletely paralysed, or it is killed outright, and thus rendered useless as food for the future larvae of the wasp. On the one hand, therefore, the Tarantula has acquired the habit of striking the wasp in the only point where its blow is instantaneously fatal, while on the other the wasp, with a different object in view, has been led to select the precise spot where its sting will disable without immediately destroying the spider. The latter case is, if anything, the more extraordinary, as the insect can hardly have any recollection of its larval tastes, and yet it stores up for progeny, which it will never see, food which is entirely abhorrent to itself in its imago state.
Spiders taken from the egg-nests of wasps by M‘Cook survived, on the average, about a fortnight, during which period they remained entirely motionless, and would retain any attitude in which they were placed.
There are many animals which either habitually or occasionally feed upon spiders. They are the staple food of some hummingbirds, and many other birds appear to find in them a pleasing variation on their customary insect diet. These creatures, moreover, are destructive to spiders in another way, by stealing the material of their webs, and especially the more closely textured silk of their egg-cocoons, to aid in the construction of their nests. M‘Cook has observed this habit in the case of Vireo noveborocensis, and he states, on the authority of others, that the “Plover” and the “Wren” are addicted to it. The smaller species of monkeys are extremely fond of spiders, and devour large numbers of them. They are said, moreover, to take a mischievous delight in pulling them in pieces. Armadillos, ant-eaters, snakes, lizards, and indeed all animals of insectivorous habit, draw no distinction between Insecta and Arachnida, but feed upon both indiscriminately. The army ants, so destructive to insect life in tropical countries, include spiders among their victims. These formidable insects 371march along in vast hordes, swarming over and tearing in pieces any small animal which lies in their path. They climb over intervening obstacles, searching every cranny, and stripping them bare of animal life. Insects which attempt to save themselves by flight are preyed upon by the birds, which are always to be seen hovering above the advancing army. The spider’s only resource is to hang from its thread in mid-air beneath the branch over which the ants are swarming, for the spider line is impracticable to the ant. Belt[294] has observed a spider escape the general destruction by this means.
Protective Coloration.—Examples are numerous in which the spider relies upon the inconspicuousness not of its nest, but of itself, to escape its natural foes. Its general hues and markings are either such as to render it not readily distinguishable among its ordinary surroundings, or the principle has been carried still further, and a special object has been “mimicked” with more or less fidelity.
This country is not rich in the more striking mimetic forms, but the observer cannot fail to notice a very general correspondence in hue between the spiders of various habits of life and their environment. Those which run on the ground are usually dull-coloured; tree-living species affect grey and green tints, and those which hunt their food amongst sand and stones are frequently so mottled with yellow, red, and grey, that they can scarcely be recognised except when in motion.
A few of our indigenous species may be mentioned as especially protected by their colour and conformation. Tibellus oblongus is a straw-coloured spider with an elongated body, which lives among dry grass and rushes. When alarmed it clings closely to a dry stem, remains motionless, and escapes observation by its peculiarity of colour and shape. Misumena vatia, another of the Thomisidae or Crab-spiders, approximates in colour to the flowers in which it is accustomed to lurk on the watch for prey. It is of a variable hue, generally yellow or pink, and some observers believe that they have seen it gently waving its anterior legs in a way which made them easily mistaken for the stamens of the flower stirred by the breeze. Its purpose appears to be to deceive, not its enemies, but its victims. It seems to be partial to the blooms of the great mullein (Verbascum thapsus), and 372Pickard-Cambridge has more than once seen it seize and overcome a bee which had visited the flower in search of honey. He has also observed it in the blossoms of rose and furze bushes.[295]
An Epeirid (Tetragnatha extensa) resembles Tibellus in its method of concealing itself when alarmed. It also possesses an elongated abdomen, of a grey-green tint, which it closely applies to one of the twigs among which it has stretched its net, at the same time extending its four long anterior legs straight before it, and in this position it lies perdu, and is very easily overlooked. Another Orb-weaver, Epeira cucurbitina, is of an apple-green colour, which is admirably calculated to conceal it among the leaves which surround its snare.
Most of our English Attidae, or Jumping-spiders, imitate closely the prevailing tone of the surfaces on which they are accustomed to hunt. This will be recognised in the familiar striped Wall-spider, Salticus scenicus, and we may also mention the grey Attus pubescens, which affects stone walls, and the speckled Attus saltator, which is hardly distinguishable from the sand which it searches for food.
Examples may also be found among the Lycosidae or Wolf-spiders. Of the prettily variegated Lycosa picta, Pickard-Cambridge says: “Much variation exists in the extent of the different portions of the pattern and in their depth of colouring, these often taking their prevailing tint from the colour of the soil in which the spider is found. The best marked, richest coloured, and largest examples are found on sandy and gravelly heaths, where there is considerable depth and variety of colouring.... But on the uniformly tinted greyish-yellow sandhills between Poole and Christchurch I have found a dwarf, pale yellow-brown variety, with scarcely any dark markings on it at all, the legs being of a uniform hue, and wholly destitute of dark annuli.”[296]
Mimicry.—In the island of Portland, a locality remarkable for the number of species peculiar to itself, there is found a spider, Micaria scintillans, very closely resembling a large blackish ant which frequents the same neighbourhood. Its movements, moreover, are exceedingly ant-like, as it hurries along in a zigzag course, frequently running up and down grass stems after the manner of those insects. It is a great lover of sunshine, and disappears as soon as the sun is obscured by a passing cloud.
373Such resemblances, obvious enough in nature, and heightened by the behaviour of the mimetic form, are often by no means striking in the cabinet. In some American species of spiders, however, imitation of the ant has passed beyond the stage of a general resemblance as regards size and colour and method of progression. The head of the ant is well marked off from the body, and the thorax is frequently divided into distinct regions. These peculiarities are imitated by constrictions in the cephalothorax of mimetic spiders. The resemblance, moreover, is much increased by their habit of using but six legs for locomotion, and carrying the second pair as ants do their antennae. The best known examples of these spiders are Synageles picata and Synemosyna formica (see Fig. 215, C, p. 420), and even more striking resemblances have been observed among some undescribed South American species.
The object of such mimicry seems to vary in different cases. Sometimes the spider preys upon the ant which it resembles. Sometimes, again, by its disguise, it escapes the notice of the ant which would otherwise feed upon it. More often spider and ant are neutral as regards each other, but, under cover of its resemblance, the Arachnid is enabled to approach an unsuspecting victim to which the ant is not a terror. Again, the unpleasantly acid taste of ants is unpalatable to most birds, though not to all, and the increased danger from specially ant-eating birds may be more than counterbalanced by the immunity they acquire from other birds.
There is quite a large class of Spiders of nocturnal habits, whose only precaution by day is to sit perfectly still and be mistaken for something else. We have referred to the adaptation in colour of our English species, Misumena vatia, to the flowers in which it lies in wait for prey. Bates[297] mentions exotic examples of the same family which mimic flower-buds in the axils of leaves. Herbert Smith says of a spider which sits upon a leaf waiting for prey: “The pink three-lobed body appears just like a withered flower that might have fallen from the tree above; to the flies, no doubt, the deception is increased by the strong sweet odour, like jasmine.”
Trimen[298] describes a Cape Town species which is of the exact rose-red of the flower of the oleander. “To more effectually conceal it, the palpi, top of the cephalothorax, and four lateral 374stripes on the abdomen are white, according remarkably with the irregular white markings so frequent on the petals of Nerium.”
The same observer, approaching a bush of the yellow-flowered Senecio pubigera, noticed that two of the numerous butterflies settled upon it did not fly away with their companions. Each of these he found to be in the clutches of a spider, whose remarkable resemblance to the flower lay not only in its colour, but in the attitude it assumed. “Holding on to the flower-stalk by the two hinder pairs of legs, it extended the two long front pairs upward and laterally. In this position it was scarcely possible to believe that it was not a flower seen in profile, the rounded abdomen representing the central mass of florets, and the extended legs the ray florets; while, to complete the illusion, the femora of the front pair of legs, adpressed to the thorax, have each a longitudinal red stripe which represents the ferruginous stripe on the sepals of the flower.”
Cambridge found in Palestine some species of Thomisidae which, when at rest, were indistinguishable from bits of coarse fleecy wool, or the rough seeds of some plant.
There is perhaps no more curious case of mimicry than that of a spider, Phrynarachne (= Ornithoscatoides) decipiens, which Forbes discovered in Java while butterfly-hunting. It appears that butterflies of the Family Hesperidae have a custom of settling, for reasons best known to themselves, upon the excreta of birds, dropped upon a leaf. Forbes noticed one in this position. Creeping up, he seized the butterfly, but found it mysteriously glued by the feet. On further investigation the “excreta” proved to be a spider. So accurate was the mimicry that he was again completely deceived by the same species in Sumatra. Its habit is to weave upon a leaf a small white patch of web, of a shape which greatly assists the deception, and in the midst of this it lies on its back, holding on by the spines with which its legs are furnished. It then folds its legs over its thorax, and waits for some insect to settle upon it.
In rare cases spiders have come to resemble their enemies the Ichneumon flies. A frequent habit of these insects is to deposit their eggs in the newly-formed cocoon of the spider. The Ichneumon eggs are the first to hatch, and the larvae have a convenient food-supply at hand. Sometimes, however, they adopt another method, and insert their eggs into the body of the spider 375itself. It is probably in order to avoid this unpleasant contingency that the spider has evinced towards the Ichneumon the sincerest form of flattery.
The Senses of Spiders.
Sight.—Though, as has been shown, spiders are well provided with eyes, their power of vision, in most cases, is by no means remarkable. As might be expected, it is less developed in those of sedentary than in those of nomadic habit.
It is noticeable that, in most spiders, some of the eyes are of a pearly grey colour, and others of a much darker hue. Simon designates the former nocturnal and the latter diurnal eyes, according to the special use which he believes them to subserve.
This view of the matter cannot be regarded as at all established, and has not found general acceptation. Moreover, Pillai[299] has shown that certain Attid spiders can change the colour of their eyes by a movement of the internal mechanism. The Epeiridae, spinners of the round web, are certainly, as a rule, very dim-sighted creatures. A fly may be held within an inch of them, but, unless it buzz, it will excite no notice whatever. A careful observation of the performances of the large Garden-spider in securing her prey will soon convince the onlooker that she is guided almost entirely by appeals to her sense of touch communicated along the tremulous lines of her snare. Interpreting these too hastily, she will sometimes rush straight past the entangled fly, and wait for it to renew its struggles before making sure of its whereabouts. Keen sight would be of little utility to such spiders, as they are concerned with nothing beyond the limits of their snare, and within its range they are furnished with the equivalent of complete telegraphic communication.
That most of the vagabond spiders can see well within the range of several inches there is no doubt, though some observers have been misled by the result of certain experiments on the Lycosidae, or “Wolf-spiders.” It will be remembered that the female Lycosid carries her egg-bag about with her, attached usually to her spinnerets. If it be removed and placed close at hand, the spider experiences the greatest difficulty in finding it again. Lubbock attributed this to defective sight, whereas it merely arises from unfamiliarity with the appearance of the 376egg-bag, which, since its construction, has been so situated as to be out of the view of the spider. Peckham found that spiders of the genus Theridion, accustomed to the sight of their cocoons, readily recognised them by that sense when removed to a distance.
The most keen-sighted of the spider tribe are undoubtedly the Attidae, or Leaping-spiders. The little black and white striped Wall-spider, Salticus scenicus, is probably a familiar object to most of our readers, and a very little observation of its movements, like those of a cat stalking a bird, will convince the observer that its visual powers are wonderfully keen and accurate. Its attitude of “attention” on sighting its prey, its stealthy manœuvring to approach it unobserved, and the unerring certainty of its final leap, are very interesting to witness.
It is somewhat noticeable that both in the Epeiridae and in the Attidae the two portions of the body, cephalothorax and abdomen, have more than the usual freedom of independent motion. In the Orb-weavers this gives play to the spinnerets in binding up a captured insect, but in the Leaping-spiders it allows of the rapid directing of the large anterior eyes towards the quarry, as it continually alters its position.
Professor and Mrs. Peckham of Wisconsin[300] performed some interesting experiments to ascertain the sensitiveness of the spider’s eye to colour. Freely communicating compartments of differently coloured glass were constructed, and spiders were confined in them, when it was found that red was the most and blue the least attractive hue. This agrees well with what Lubbock found to be the case with ants, but those insects displayed a greater antipathy for blue and not so marked a preference for red.
Hearing.—Most of our knowledge about the auditory sense of spiders is due to experiments performed by C. V. Boys,[301] and repeated by Professor and Mrs. Peckham.
The spider usually responds to the stimulus in one of two ways; it either raises its front legs, extending them in the direction of the sound, or it allows itself to drop suddenly, as though in alarm. It was only in the case of the Epeiridae that any results were obtained, and these spiders were more sensitive to low than to high notes. Now, as M‘Cook points out, it is 377exceedingly strange that the nomadic and hunting spiders, to which the sense of hearing might be expected to be extremely useful, should be deficient in this faculty, while the sedentary spiders, to which it would appear comparatively unimportant, should possess it in a tolerably developed form. That writer may possibly be correct in supposing that the sense, as possessed by spiders, is hardly differentiated from that of ordinary touch, and that the web-making species are only aware of sounds by the vibrations communicated to their feet by the medium of the web. However this may be, we must reluctantly but sternly reject the numerous and seemingly authentic stories, often connected with historic personages, which credit the spider with a cultivated taste for music.
We have seen that among the spiders which possess a stridulating apparatus it is confined, in certain groups, to the male, or if present in the female it exists only in a rudimentary form. If in these cases stridulation has been rightly interpreted as a sexual call, the power of hearing, at least in the female, is of course connoted. The spiders in question are members of the Theridiidae, a family closely allied to the Epeiridae, and therefore more likely than most groups to possess the power of hearing.
Theraphosid spiders show no response to the stimulus of sound, and among them stridulation is not confined to one sex. If, as is generally believed, the organ is used to warn off enemies, it is not necessary that the sound produced should be audible to the spider itself. If there be any true hearing organ in spiders its location is quite uncertain. Some have supposed the so-called lyriform organs in the legs to have an auditory function, while others have supposed the power of hearing to reside in certain hairs, of which there are several different types distributed over the body and limbs of the animal.
Spider Intelligence.—The experiments performed by the Peckhams clearly proved that spiders have short memories—a sure indication of a low state of intelligence. Members of the Lycosid or “Wolf-spider” group, when deprived of their cocoons, recognised them again after a few hours, but in most instances they refused to resume them after a lapse of twenty-four hours, and in every case an absence of two days sufficed to prevent any sign of recognition on their restoration. Moreover, when, after a shorter interval, the cocoons of other spiders, even of different 378genera, were offered to them, they appeared equally satisfied, and attached them in the orthodox manner, beneath the abdomen. The same treatment was even accorded to pith balls, which, if of the right size, seemed to be a perfectly satisfactory substitute. The contents of one cocoon were replaced by a shot three or four times their weight, but the spider accepted it with alacrity, spending half an hour in refixing it, when its weight caused it to fall from its attachment.
The habit of “feigning death,” which seems to be especially characteristic of the Epeiridae or orb-weaving spiders, probably arises from no desire to deceive its adversary as to its condition, but from an instinct to remain motionless, and therefore inconspicuous. Where a nomadic spider seeks safety in flight, a sedentary species finds a greater chance of escape in dropping a certain distance, and, while still attached by its silken line, giving as little evidence of its whereabouts as possible—trusting, in many cases, to its protective colouring. This method, moreover, has the advantage of facilitating its return to the web when the danger is past—a feat of which it would be quite incapable were it once to relinquish its clue.
All the remarkable and apparently intelligent actions of these creatures seem to be done in obedience to a blind instinct, which is obeyed even when there is no longer any object to be served. We have seen how the Trap-door spiders decorate the lids of their nests with moss even when the surrounding ground is bare, and Agelena labyrinthica has been observed to go through the whole lengthy and laborious operation of constructing its egg-cocoon though all its eggs were removed immediately on being laid.[302]
Mating Habits.—The sex of a mature spider can readily be recognised by the palpus which, as we have seen, is furnished in the male with a “palpal organ.” After the last moult but one the palp appears tumid, but it is only at the last moult that the organ is fully formed, and that the genital orifice is visible under the anterior part of the abdomen.
No alteration takes place in the female palp at maturity, but it is only after the last moult that the “epigyne” is distinguishable.

Fig. 198.—Argiope aurelia, ♂ and ♀, natural size.
That the palpal organs are used in the fertilisation of the female has long been established. How they came to contain 379the sperm matured in the abdomen was a problem which has only been solved comparatively recently. No direct connection could be found by way of the palpus with the abdominal organs, which, indeed, were seen to have an orifice between the lung-sacs. It is now known that some spiders at all events spin a slight web upon which they deposit a drop of spermatic fluid, which they afterwards absorb into their palpal organs for transference to the female. Secondary sexual differences are often very marked, the male being almost invariably the smaller in body, though its legs are frequently longer and more powerful than those of the female.
Among some of the sedentary spiders the disparity in size is excessive. The most striking examples are furnished by the Epeirid genera Argiope and Nephila, the male in some instances not attaining more than the thousandth part of the mass of the female. The coloration of the sexes is frequently quite dissimilar, the male being usually the darker, though in the Attidae he is in many cases the more strikingly ornamented.
In the minute Theridiid spiders of the group Erigoninae (see p. 404), the male cephalothorax often presents remarkable and characteristic excrescences not observable in the female. Some curious examples of this phenomenon may be seen in Fig. 209.
To the ordinary observer male spiders will appear to be comparatively rare, and to be greatly outnumbered by the females. This is probably to some degree true, but the unsettled habits of the males and the shorter duration of their life are calculated to give an exaggerated impression of their rarity. They only appear in considerable numbers at the mating season, shortly after which the males, in the case of many species, may be sought for in vain, as, after performing their functions, they quickly die. The snares they spin are often rudimentary, their capabilities in this direction appearing to deteriorate after the adult form is attained. Young spiders of indistinguishable sex make perfect snares on a small 380scale, while such as eventually develop male organs will often thereafter be content with a few straggling lines made with very slight regard to symmetry. They become nomadic in their habits, wandering off in search of the females, and pitching a hasty tent by the way.
The relations between the sexes in the Spider tribe present points of extreme interest, but in this connexion the various groups must be separately treated on account of their very different habits of life.
In no group are these relations more curious than in the Epeiridae, the constructors of the familiar wheel-like web. Love-making is no trifling matter here. If the female is not in the mood for the advances of the male she will probably regard him as a desirable addition to her larder. Even if his wooing is accepted, he has to beat a precipitate retreat after effecting his purpose, or he may fall a victim to his partner’s hunger.
This strange peril braved by the male in courting the female, which has, as far as is known, no parallel in any other department of the animal kingdom, is frequently mentioned as universal among spiders. It unquestionably exists, and may be verified by any patient observer in the case of the large Garden-spider Epeira diademata, but it has only been observed among certain species of the Epeiridae and Attidae. It will be remembered that in the Epeiridae the males are sometimes absurdly small in comparison with the females, and this diminution of size is thought to have a direct connection with the danger undergone at the mating season. Small active males stand a better chance of escape from ferocious females, so that natural selection has acted in the direction of reducing their size as far as is compatible with the performance of their functions.
Pickard-Cambridge[303] cites an extreme case. He says: “The female of Nephila chrysogaster, Walck. (an almost universally distributed tropical Epeirid), measures 2 inches in the length of its body, while that of the male scarcely exceeds ⅒th of an inch, and is less than ¹⁄₁₃₀₀th part of her weight.”
During the mating season the males may be looked for on the borders of the snares of the females. Their action is hesitating and irresolute, as it well may be, and for hours they will linger on the confines of the web, feeling it cautiously with their 381legs, and apparently trying to ascertain the nature of the welcome likely to be extended to them. If accepted, they accomplish their purpose by applying their palps alternately to the epigyne of their mate. If repulsed, they do their best to make their escape, and wait for a more auspicious moment. Emerton[304] says: “In these encounters the males are often injured; they frequently lose some of their legs; and I have seen one, that had only four out of his eight left, still standing up to his work.”
Among the other groups of sedentary spiders the relations between the sexes seem to be more pacific, and there is even some approach to domesticity. Males and females of Linyphia may be found during the mating season living happily together in their irregular snares. The same harmony seems to exist among the Tube-weavers, and Agelena labyrinthica lingers for days unmolested about the web of the female, though it is perhaps hardly correct to say that they have their home in common.
Among the wandering spiders the male usually seeks out the female and leaps on her back, from which position his sperm-laden palps can reach their destination. This is the habit of the Thomisidae or Crab-spiders, and of the quick-running Wolf-spiders, or Lycosidae.

Fig. 199.—Male Astia vittata dancing before the female. (After Peckham.)
The sexual relations of the Leaping-spiders, or Attidae, are so remarkable as to deserve a longer notice. This Family includes the most beautiful and highly ornamented examples of spider life. Their headquarters are the tropics, and their brilliant colouring led Wallace to speak of those he saw in the Malay Archipelago as “perfect gems of beauty.”
Now among these spiders the male is almost always more highly decorated than the female, and Peckham’s observations would lead to the conclusion that the female is influenced by the display of these decorations in the selection of her mate.
The so-called “love-dances” of certain tropical birds are known to all readers of natural history, 382but it was hardly to be expected that their counterpart would exist among spiders. Yet the antics by which male Attidae endeavour to attract the attention of the females afford an almost exact parallel.
The following extract from the account of Professor and Mrs. Peckham[305] of their observations on Saitis pulex will make this abundantly clear: “When some four inches from her he stood still, and then began the most remarkable performances that an amorous male could offer to an admiring female. She eyed him eagerly, changing her position from time to time, so that he might be always in view. He, raising his whole body on one side by straightening out the legs, and lowering it on the other by folding the first two pairs of legs up and under, leaned so far over as to be in danger of losing his balance, which he only maintained by sidling rapidly towards the lowered side.... Again and again he circles from side to side, she gazing towards him in a softer mood, evidently admiring the grace of his antics. This is repeated until we have counted a hundred and eleven circles made by the ardent little male. Now he approaches nearer and nearer, and when almost within reach whirls madly around and around her, she joining with him in a giddy maze. Again he falls back and resumes his semicircular motions, with his body tilted over; she, all excitement, lowers her head and raises her body so that it is almost vertical; both draw nearer; she moves slowly under him, he crawling over her head, and the mating is accomplished.”

Fig. 200.—Dancing attitude of male Icius mitratus. (After Peckham.)
A similar but not exactly identical performance was gone through by the male of several different species, but it was noteworthy that the particular attitudes he adopted were always such as to display to the best advantage his special beauties, whether they consisted in crested head, fringed palpi and fore-legs, or iridescent abdomen. Sometimes even such exertions failed to captivate the female, and she would savagely attack the male, occasionally with fatal effect.
383In the case of some species, when the male had won the consent of his mate, he would weave a small nuptial tent or web, into which he would partly lead and partly drive the female, who no longer offered serious resistance.
Fossil Spiders.
About 250 species of fossil spiders have been discovered. Of these about 180 are embedded in amber, a fossil resinous substance which exuded from ancient coniferous trees, and quantities of which are annually washed up from the Baltic upon the shores of northern Prussia.
The most ancient fossil spider known was obtained from the argillaceous slate of Kattowitz in Silesia, and belongs, therefore, to the Carboniferous strata of the Palaeozoic epoch. It has been named Protolycosa anthrocophila. There is some doubt as to the affinities of this spider. Roemer, who described it, placed it among the Citigradae, while others have thought it to belong rather to the Territelariae. Thorell, on account of its agreement in certain important points with the very curious recent Malay spider Liphistius, has placed them both in a separate sub-family, Liphistioidae. To the same epoch belongs the American fossil spider Arthrolycosa antiqua, which was found in the Coal measures of Illinois.
The other localities from which fossil spiders have been obtained are the Swiss Miocene at Oeningen, the Oligocene deposits at Aix, the Oligocene of Florissant, Colorado, Green River, Wyoming, and Quesnel, British Columbia.
Many of the spiders from the rocks are so fragmentary that it is impossible to decide with certainty on their systematic position, but a considerable number of them—more than half—have been assigned to recent genera.
The amber spiders are mostly well preserved, and can be classified with more certainty. Many of them are surprisingly like existing forms, though others, like Archaea paradoxa, differ greatly from most spiders now extant, though they show some affinities with one or two remarkable and aberrant forms.
CHAPTER XV
ARACHNIDA EMBOLOBRANCHIATA (CONTINUED)—ARANEAE (CONTINUED)—CLASSIFICATION
The systematic study of Spiders has hitherto presented very great difficulties. There is an extensive literature on the subject, but the more important works are costly, not commonly to be found in libraries, and written in diverse languages. Moreover, the nomenclature is only now emerging from a condition of chaos. Able and diligent Arachnologists have done admirable work in studying and describing the Spider fauna of their various countries, and occasional tentative suggestions have been put forth with a view to reducing to some sort of order the vast mass of heterogeneous material thus collected. Most schemes of classification, based chiefly upon a knowledge of European forms, have proved quite inadequate for the reception of the vast numbers of strange exotic species with which recent years have made us acquainted. The number of described species is very large, and is rapidly increasing; but though we are very far indeed from anything like an exhaustive knowledge of existing forms, it may now be said that almost every considerable area of the earth’s surface is at least partially represented in the cabinets of collectors, and it is possible to take a comprehensive view of the whole Spider fauna, and to suggest a scheme of classification very much less likely than heretofore to be fundamentally deranged by new discoveries.
The first to apply the Linnaean nomenclature to Spiders was Clerck, in his Araneae Suecicae (1757), which gives an account of seventy spiders, some of which are varieties of the same species. A few new species were added by Linnaeus, De Geer, Scopoli, Fabricius, etc., but the next work of real importance was that of Westring (1861), who, under the same title, described 385308 species, divided among six families. Blackwall’s beautiful work, the Spiders of Great Britain and Ireland, was published by the Ray Society in 1864. He divides spiders into three tribes, Octonoculina, Senoculina, and Binoculina, according to the number of the eyes, and describes 304 British species, distributed among eleven families.
His successor in this country has been Pickard-Cambridge, whose work, under the modest title of The Spiders of Dorset (1879–81), is indispensable to British collectors.
Blackwall’s division of the order into tribes was evidently artificial, and has not been followed by later Arachnologists. Dufour (1820) founded two sub-orders, Dipneumones and Tetrapneumones, based on the presence of two or four pulmonary sacs. Latreille (1825) established, and many Arachnologists adopted, a division into tribes based upon habits, Orbitelariae, Retitelariae, Citigradae, Latigradae, etc., and this method of classification was followed in the important work of Menge, entitled Preussische Spinnen, which was published between 1866 and 1874.
Since 1870 determined efforts have been made to grapple with the difficult subject of Spider classification, notably by Thorell and Simon. The latter, undoubtedly the foremost living Arachnologist, writes with especial authority, and it is inevitable that he should be largely followed by students of Arachnology, who cannot pretend to anything like the same width of outlook.
It is indicative of the transition stage through which the subject is passing that Simon in his two most important works,[306] propounds somewhat different schemes of classification, while in the Histoire naturelle, where his latest views are to be found, he introduces in the course of the work quite considerable modifications of the scheme set forth in the first volume.
In that work the order is divided into two sub-orders, Araneae theraphosae and Araneae verae, the first sub-order containing Liphistius and the Mygalidae or Theraphosidae of other authors, while all other spiders fall under the second sub-order. The Araneae verae are subdivided into Cribellatae and Ecribellatae, according to the presence or absence of “cribellum” and “calamistrum” (see p. 326) in the female. Important as these organs doubtless are, the Cribellatae do not appear to form 386a natural group, some of the families having apparently much closer affinities with certain of the Ecribellatae than with one another. This is especially evident in the case of the cribellate Oecobiidae and the ecribellate Urocteidae (see p. 392), which most authors unite in a single family.
After all, the larger divisions of the order are not of great importance, and in the present chapter Simon’s linear arrangement of families will in the main be followed, except for the distribution of the eight families which constitute his Cribellatae[307] to the positions which a more general view of their structure would seem to indicate.
Fam. 1. Liphistiidae.—Spiders with segmented abdomen, as shown by the presence of a series of tergal plates. Eight spinnerets in the middle of the ventral surface of the abdomen, far removed from the anal tubercle. Sternum long and narrow. Eight compact eyes on a small eminence. Four pulmonary stigmata.

Fig. 201.—Profile (nat. size) and ocular area (enlarged) of Liphistius desultor.
This Family includes a single genus and two species of large spiders (about two inches in length), one from Penang and one from Sumatra. Very few examples have been found, and these are more or less defective and in bad condition. In some respects, especially the distinct segmentation of the abdomen, this genus much more nearly approaches the Pedipalpi than do any others of the order. No other spider possesses more than six spinning mammillae, but it is possible that eight was the more primitive number, and that the “cribellum” (see p. 326) of the so-called Cribellate spiders is derived from the pair now possessed by Liphistius alone.
Some Arachnologists consider the genus Liphistius so different from all other spiders as to constitute in itself a sub-order, for which, on account of the position of its spinnerets, the name MESOTHELAE has been suggested, all other forms falling into the sub-order OPISTHOTHELAE.
Fam. 2. Aviculariidae. (Mygalidae).[308]—Spiders with independent 387chelicerae, the paturon directed forward and the unguis or fang articulating in a vertical plane. The eyes are eight (except Masteria, six), usually compact, and situated on an eminence. Pedipalpi very leg-like, and palpal organs of male simple. No maxillae. Four pulmonary stigmata. Spinnerets normally four. No colulus.
The Aviculariidae inhabit the warmer portions of the world, and are entirely unrepresented in this country. The monster spiders which excite wonder in zoological collections belong to this group, as do the moderate-sized “Trap-door” Spiders which are found abundantly in the Mediterranean region.
The Family has been divided into about a hundred and fifty genera, nearly half of which, however, contain only a single species.
They have been grouped by Simon[309] into seven sub-families, Paratropidinae, Actinopodinae, Miginae, Ctenizinae, Barychelinae, Aviculariinae, and Diplurinae, of which the first three may be dealt with very briefly.
(i.) The Paratropidinae include only two American species, Paratropis scrupea from the Amazon, and Anisaspis bacillifera from St. Vincent. They have thick, rugose integuments, and the internal angle of the coxa of the pedipalp is produced. The labium is fused with the sternum, which is very broad. Nothing is known of their habits, but as they do not possess a “rastellus” (see p. 320) they are probably not burrowing spiders.
(ii.) The Actinopodinae comprise three genera, Stasinopus represented by a single South African species, S. caffrus; Eriodon, of which about ten species inhabit Australia; and Actinopus, of which about ten species are found in Central and South America. They have the coxae of the pedipalps very short and broad, and somewhat produced at the internal angle. The eyes are not in the usual compact group, but are somewhat extended across the caput. Actinopus burrows a deep cylindrical hole lined with silk, and furnished with a round, bevelled trap-door.
(iii.) The sub-family Miginae is established for the reception of three genera, Moggridgea (South Africa), Migas (Australia and South-West Africa), and Myrtale, whose single species, M. perroti, inhabits Madagascar. They are chiefly characterised by their very short and downwardly-directed chelicerae. They are not 388terricolous, but inhabit trees, either boring holes in the bark, or constructing a sort of silken retreat fortified by particles of wood.
(iv.) The Ctenizinae form a large group, including some forty genera. All the “Trap-door” Spiders of the Continent fall under this sub-family, which, moreover, has representatives in all the tropical and sub-tropical regions of the world. A rastellus is always present, and the eyes form a compact group on an eminence. The coxae of the pedipalps are longer than in the groups previously mentioned, and there is no production of the internal angle. The labium is generally free.
The commonest European genus is Nemesia, of which about thirty species inhabit the Mediterranean region. The cephalothorax is rather flat, and the central fovea is recurved (◠). The burrow is sometimes simple and sometimes branched, and the trap-door may be either thin, or thick with bevelled edges.
Allied genera are Hermacha and Rachias in South America, Spiroctenus in South Africa, Genysa in Madagascar, Scalidognathus in Ceylon, and Arbanitis in New Zealand. The genus Cteniza (fovea procurved ◡) possesses only a single species (C. sauvagei), found in South-East France and Italy.
Pachylomerus is a widely-distributed genus, being represented in North and South America, Japan, and North Africa. The tibiae of the third pair of legs are marked above by a deep impression near the base. A closely allied genus, Conothele. inhabits Southern Asia and New Guinea.
The widely-distributed genus Acanthodon, which has representatives in all the sub-tropical countries of the world, together with the South American genera Idiops and Pseudidiops, and the Indian genus Heligmonerus, present a peculiar arrangement of the eyes, one pair being situated close together in the middle of the front of the caput, while the remaining six form a more or less compact group some distance behind them.
Among the many other genera of the Ctenizinae may be mentioned Cyrtauchenius, of which many species inhabit North-West Africa, and its close ally Amblyocarenum, represented on both shores of the Mediterranean, and in North and South America. They differ from Cteniza chiefly in the possession of strong scopulae on the tarsi and metatarsi of the first pair of legs, and in the double row of teeth with which the tarsal claws are furnished. 389Their burrows are often surmounted by a sort of turret raised above the level of the ground.
(v.) The Barychelinae are burrowing forms which resemble Nemesia, but have only two tarsal claws. Leptopelma is the only European genus, and has close affinities with certain South American genera (Psalistops, Euthycoelus, etc.). Pisenor inhabits tropical Africa, and Diplothele, unique in possessing only two spinning mammillae, is an inhabitant of India.
(vi.) The Aviculariinae include all the large hairy spiders which are commonly called Mygale. The genus Phlogius, which inhabits Southern Asia, forms a lidless burrow, though it has no rastellus, but practically all the other members of the group are non-terricolous, living under stones or in holes in trees, where they weave a slight web. They are nocturnal in their habits. They all possess two tarsal claws, and the labium is free and spined at the tip. Of the four spinnerets the posterior pair are long and three-jointed, while the anterior are short and not very close together.
The particular form of the tarsi and the nature of the scopulae,[310] “claw-tufts,” and spines upon them are of great importance in distinguishing the members of this group.
The Aviculariinae comprise about sixty genera from all the tropical and sub-tropical regions of the world.
The genus Ischnocolus extends into the Mediterranean region, having representatives besides in Southern Asia and in Central and South America. All the tarsi have their scopulae divided longitudinally by a band of hairs. Chaetopelma inhabits Egypt, Syria, and Arabia, and Cyclosternum is found in West Africa as well as in Central and South America. In these genera the scopulae of the last two pairs of legs are alone divided. The largest known spider is Theraphosa leblondi, which is a native of Guiana. It measures 9 cm. (about three and a half inches) in length.
Eurypelma is a genus of large spiders entirely confined to the New World, where it possesses many species. The genus Avicularia is also American, and includes a number of large long-haired spiders with short and very strong legs, on which 390the scopulae and claw-tufts are well developed. Its nearest allies in the Old World are the Indian genus Poecilotheria, and the West African genus Scodra. The stridulating spider figured on p. 328 belongs to this group, Chilobrachys being a genus from Ceylon.

Fig. 202.—Ischnothele dumicola, ♀ × 2. (After Pocock.)
(vii.) The Diplurinae are a very aberrant group, including some twenty genera of Aviculariidae, usually of medium size, and possessed, as a rule, of very long posterior spinnerets. They do not burrow or live in holes or under stones, but weave webs of close texture, much resembling those characteristic of the Agelenidae (see p. 415). The tarsal claws are three in number, and there are never any claw-tufts. The rastellus, of course, is absent.
Two genera have representatives in Europe, Brachythele inhabiting the East Mediterranean region (as well as many other parts of the world), while Macrothele is found in Spain as well as in the Malay Peninsula and New Zealand. Ischnothele dumicola is a native of Western India. Diplura is a South American genus. Trechona venosa, a large species remarkable for the orange bands which decorate its abdomen, is also a native of South America. The New Zealand genus Hexathele, and the genus Scotinoecus from Chili, possess six spinnerets. Masteria (Ovalan Island) and Accola (Philippines and South America) differ from the rest of the family in having only six eyes.
Fam. 3. Atypidae.—Spiders with anteriorly projecting and vertically articulating chelicerae, but with no trough on the paturon for the reception of the unguis, which is guarded when closed by a single row of teeth. The spinnerets are normally six, and the anal tubercle is above, and well removed from the posterior spinnerets.

Fig. 203.—Atypus affinis, ♀.
The Atypidae are a small family of six genera, rather closely related to the Aviculariidae, and by some Arachnologists incorporated with them. They may be regarded as the representatives of that family in sub-tropical and temperate regions. In form they are strongly built, with smooth integuments, and their legs are short and powerful. Of the twenty-four species hitherto 391described almost all belong to the northern hemisphere. Five are natives of Europe, and two are included in the English fauna. The best known is Atypus affinis, which has been found in several localities in the south of England, and which has occurred on the Devil’s Dyke, near Cambridge. The female measures about half an inch in length, the male being smaller. It burrows a deep cylindrical hole at the edge of a grassy or heathery bank and lines it with a loose tube of silk, which extends considerably beyond the orifice of the burrow, either lying flat on the ground, or raised up and attached to the neighbouring herbage. There is no lid, but the upper end of the tube is always found closed, whether by its elasticity or by the deliberate operation of the spider is not known. The animal is nocturnal in its habits. Another species, A. beckii, occurs very rarely in the south of England.
The genus Atypus has representatives in Central and South Europe, North Africa, Japan, Java, and North America. Of the other genera, Calommata inhabits Central and South-East Asia and Japan, Brachybothrium, Atypoides, and Hexura are peculiar to North America, while Mecicobothrium comprises a single species (M. thorelli) native to the Argentine.[311]
Fam. 4. Filistatidae.—Cribellate Spiders of moderate size, usually brown or yellow in colour, with smooth integuments and somewhat long tapering legs. The eight eyes are compactly arranged, and the palpal organs of the male are of simple structure. The six spinnerets are short, the anterior pair being thick and separated. Two pulmonary sacs, with two minute tracheal stigmata close behind them and widely separate.
There is but one genus, Filistata, in this family. About fifteen species have been described, five of which inhabit the Mediterranean region. Three are found in America, and others inhabit Central Asia, the Philippines, and Australia. The genus 392is not represented in this country, but one species, F. testacea, has an extremely wide distribution in the Old World, while F. capitata extends throughout the American continent.
The calamistrum of the female is short, only occupying a portion of the metatarsus of the fourth leg. The cribellum is divided. These spiders weave a web of close texture, of an irregular tubular form.
Fam. 5. Oecobiidae (Urocteidae).—Two very remarkable genera constitute this family, Oecobius and Uroctea.
The species of Oecobius, about fifteen in number, are small spiders, inhabiting sub-tropical countries—and especially desert regions—and spinning a slight web under stones, or in holes in walls. The female possesses a small transverse cribellum, the two halves of which are widely separated. The calamistrum is but feebly developed. No example has occurred in this country, but nine species have been described in the Mediterranean region.

Fig. 204.—A, Oecobius maculatus, much enlarged; B, Uroctea durandi, slightly enlarged. (After Simon.)
The three species of Uroctea are rather large spiders, two being native to Africa, while the third inhabits China and Japan. They are ecribellate. These two genera very closely resemble each other, not only superficially, but in certain structural details—notably the remarkably developed and two-jointed anal tubercle—and their close affinity supplies the strongest argument against separating the spiders which possess cribellum and calamistrum into a group by themselves. In both genera the cephalothorax is very broad and rounded at the sides. The eight eyes are compactly arranged. The sternum is broad and heart-shaped. 393The legs are nearly of equal length, and the posterior spinnerets have very long terminal joints.
Fam. 6. Sicariidae (Scytodidae).—The Sicariidae are a small group of six-eyed spiders, usually with weak legs and slow halting movements; they live under stones or in outhouses. The cephalothorax is generally smooth and devoid of the median fovea, and the palpal organs of the male are extremely simple. The best known genus is Scytodes, one species of which (S. thoracica) has on rare occasions been found in outhouses in the south of England, in Dorsetshire, and Kent. This is a remarkable spider, about one-third of an inch long, with a pale yellow ground-colour, marked with black spots and patches. The cephalothorax is smooth and dome-shaped, and highest near the posterior end.
All the other members of the family are exotic. Loxosceles is found in the Mediterranean region and all over America, as well as in Japan. The median fovea is present in this genus. Sicarius is a native of America and South Africa. It is of stouter build than Scytodes, and the legs are stronger. Drymusa belongs to South Africa. The peculiar New Zealand species Periegops hirsutus is placed by Simon in this family, as is also the North American genus Plectreurys, notwithstanding its possession of eight eyes.
Fam. 7. Hypochilidae.—Two species only are included in this family, Hypochilus thorelli of North America, and Ectatosticta davidi, a native of China. They have four pulmonary sacs, though they possess little else in common with the “Theraphosae.” The pedipalpus of the male is very remarkable, the tarsus being almost unmodified, and the very small palpal organ being inserted at its extremity. These spiders are cribellate.
Fam. 8. Leptonetidae.—The Leptonetidae are small spiders with smooth and usually dull-coloured integuments. Most of them are cave-living, but some are found amidst vegetable débris in damp spots in forests. The eyes are six in number, and the legs are generally long and thin. There are five genera. Leptoneta has about ten species living in caves in the Pyrenees. The single species of Telema (T. tenella) has the same habitat. Ochyrocera has representatives in tropical Asia and America, and is somewhat more ornate than most members of the group. Usofila has a single species, inhabiting North America, while Theotina is found in caves in the Philippines and in Venezuela.
Fam. 9. Oonopidae.—The Oonopidae are very small spiders, 394seldom exceeding 2 mm. in length (the largest 4 mm.), living among vegetable débris. Oonops pulcher, the only English representative of the family, is not rare under stones or in the débris at the bottom of hedges. It is a small brick-red spider, easily recognised by its six comparatively large oval eyes, which are pale-coloured, and occupy the whole of the caput.
The minute spiders of this family were until recently overlooked by collectors in foreign countries, but now more than a hundred species have been described, belonging to some eighteen genera. Thirteen species inhabit the Mediterranean region, occurring especially on the African side. In several genera there is a “scutum” or hard plate on the abdomen. This is the case with Dysderina, which has a wide distribution, as have also Ischnyothyreus and Opopaea, and the non-scutate genus Orchestina.
Fam. 10. Hadrotarsidae.—This family contains only two species, Hadrotarsus babirusa from New Guinea, and Gmogala scarabeus from Sydney. In general appearance they resemble the scutate Oonopidae, but they have eight eyes, curiously arranged, two large, somewhat triangular eyes being situated near the middle of the cephalothorax, and two groups of three small eyes on either side of the front part of the caput. These spiders are very minute.
Fam. 11. Dysderidae.—Six-eyed spiders, with long free labium, and long maxillae provided with a well-developed scopula. The cephalothorax is rather flat, and the abdomen is oval or cylindrical, the integument being smooth and usually rather soft. The palpal organ of the male is of simple structure.
The Dysderidae are divided into two sub-families, Dysderinae and Segestriinae, for the most part confined to temperate regions.
(i.) The Dysderinae are easily recognised by a peculiarity of the sternum. Instead of being merely excavated along its border for the reception of the legs, its edge is folded round the coxae to meet the carapace, and thus forms a series of collars or sockets in which the limbs are articulated in perfect isolation from each other. These spiders vary considerably in size, and are generally of a somewhat uniform coloration, never marked with vivid patterns. There are eight genera of this sub-family, two of which are represented in England.
Dysdera cambridgii is not a rare spider under stones in rocky 395localities, such as the Isle of Portland, and occurs, though less commonly, all over the country in similar situations, and under the loose bark of trees. It is half an inch in length, with a chestnut-coloured cephalothorax and legs, and dull yellow abdomen. A closely allied species, D. crocota, also occurs more rarely.
Harpactes hombergii is common in vegetable débris and under decaying bark. It is about a quarter of an inch in length, of slender form, with black-brown cephalothorax and clay-coloured abdomen. The legs are yellowish and annulated. More than forty exotic species of Dysdera and twenty-four of Harpactes have been described. Another genus of the Dysderinae is Stalita, which comprises three species, inhabiting the caves of Dalmatia and Carniola.
(ii.) The Segestriinae include two genera, Segestria and Ariadna.
Segestria senoculata occurs in England in similar localities to those where Dysdera cambridgii is found. It is not much smaller than that spider, and has a dark brown cephalothorax and legs and a dull yellow abdomen, with a series of adder-like diamond-shaped black markings along the middle. Two other species have occurred on rare occasions in England, and twelve more are recorded from the various temperate regions of the world.
Ariadna is the only Dysderid genus which invades the tropical regions. It includes about twenty species.
Fam. 12. Caponiidae.—This is a small family of three genera and about twelve species, remarkable in having no pulmonary sacs but five tracheal stigmata,[312] and in the peculiar arrangement of their six spinnerets, those which are ordinarily median being in the same transverse line with the anterior ones.
The single species of Caponia (C. natalensis) inhabits South Africa, while Caponina has two species in South America. These spiders are eight-eyed, but the two median posterior eyes are much the largest, and these alone are present in the remarkable genus Nops, of which several species inhabit South America and adjacent islands.
Fam. 13. Prodidomidae.—This small family includes about 396twenty species of minute spiders from sub-tropical regions. They are eight-eyed, with short smooth legs, terminated by two claws not dentated. The spinnerets are especially characteristic.
Prodidomus (Miltia) includes fifteen species from the Mediterranean region, Africa, and America. Zimris is an Asiatic genus. The single species of Eleleis (E. crinita) is from the Cape.

Fig. 205.—Drassid Spiders. 1. Drassus lapidosus. 2. Clubiona corticalis. 3. Zora spinimana. 4. Micaria pulicaria.
Fam. 14. Drassidae.—Elongate spiders with low cephalothorax. Legs usually rather long, strong, and tapering, terminated by two pectinate claws, armed with spines, and scopulate. The body is smooth or short haired and frequently unicolorous and sombre-coloured, seldom ornate. The eyes, normally eight, are in two transverse rows. The mouth-parts (labium and maxillae) are long. Spinnerets as a rule terminal, and visible from above.
This important family includes a large number of species from all parts of the world, fifty-six being natives of the British Isles. There are familiar examples in the brown or mouse-coloured spiders which scurry away when stones are raised, or when loose bark is pulled off a tree.
The family may be divided into seven sub-families, of which four, Drassinae, Clubioninae, Liocraninae, and Micariinae, are represented in this country.
(i.) The Drassinae include more than twenty genera, some of which possess numerous species and have a wide distribution. The following may be mentioned:—
397Drassus contains twelve British species. The commonest is D. lapidosus, a large dull brown spider, more than half an inch in length, which lives beneath stones in all parts of the country. At least a hundred species of this genus have been described.
Melanophora (= Prosthesima)[313] includes a large number of species. They are dark-coloured active spiders, many of them jet black and glossy. Seven are recorded from the British Isles, the average size being about a quarter of an inch. They are found under stones. A closely allied genus is Phaeocedus, whose single species (P. braccatus) has occurred, though very rarely, in the south of England. Gnaphosa has fifty-five species, of which twenty-eight are European, and four are British.
(ii.) The Clubioninae have the anterior spinnerets closer together, and the eyes more extended across the caput than in the foregoing sub-family. Nearly thirty genera have been established, of which three claim special attention. Clubiona includes more than 100 species, chiefly inhabiting temperate regions. Fifteen are included in the British list. They are mostly unicolorous, and yellow or brown in colour, but a few (C. corticalis, C. compta, etc.) have a distinct pattern on the abdomen. Cheiracanthium is a large and widely spread genus, counting three English species. There are more than a hundred species of the genus Anyphaena, of which one only (A. accentuata) occurs in this country, where it is common upon bushes and trees in the south.
(iii.) The Liocraninae include about twenty-four genera, of which Zora, Liocranum, Agroeca, and Micariosoma are sparingly represented in this country.
(iv.) The Micariinae are a remarkable group of Spiders containing numerous ant-like mimetic forms. Two species of Micaria alone are English, but that genus is abundantly represented on the Continent, where the species mount up to forty. They are mostly small, dark, shining spiders, which, though not particularly ant-like in form, recall those insects both by their appearance and movements. Some of the exotic genera, and particularly the South American genus Myrmecium, possess remarkable instances of mimetic resemblance to ants. Micaria pulicaria is a very 398pretty little spider, about a sixth of an inch in length, black, with iridescent hairs, and some white marks on the abdomen. It runs about in a very active ant-like fashion and does not object to the sunshine. It is fairly abundant in England.
Fam. 15. Palpimanidae.—This family includes a few genera of exotic spiders. They are especially characterised by the great development of their anterior legs, which are not much used for locomotion, but are frequently raised as the spider moves along, generally somewhat slowly, by means of the other three pairs. The best known genera are Metronax and Stenochilus from India, Huttonia from New Zealand, and Palpimanus from the Mediterranean region, Africa, and South Asia.
Fam. 16. Eresidae.—The Eresidae are a small family of cribellate spiders whose systematic position has been the subject of much discussion. In general appearance they resemble the Attidae (vide infra), but this resemblance is quite superficial. On the whole they seem more nearly allied to the following family than to any other. They are stoutly built, with thick, strong legs, and live either in the ground or on bushes, where they weave a close-textured web. One species, Eresus cinnaberinus, has occurred on rare occasions in the south of England, and the male, which is a third of an inch in length, is perhaps the most striking member of our Spider fauna, the abdomen being scarlet, with four (or sometimes six) black spots edged with white hairs. The cephalothorax is black, with red on the postero-lateral borders. The abdomen of the female is black.
Fam. 17. Dictynidae.—Cribellate spiders, with oval cephalothorax and broad convex caput, with the eyes, normally eight, ranged across it in two straight or slightly curved transverse rows. Basal joints of chelicerae long and strong, often bowed. Legs rather strong. Tarsi three-clawed and devoid of scopula.
The Dictynidae are sedentary spiders which weave a web of irregular strands, covered by the close weft which is the product of the cribellum. Some live under stones or in holes in walls, while others spin their webs in bushes or herbage. There are about sixteen genera, of which Dictyna and Amaurobius are the most important.
Nearly a hundred species of Dictyna have been described. They are small spiders, usually living in grass and herbage. Thirty species inhabit Europe and the neighbouring coast of 399Africa, and eight of these are natives of Britain. D. arundinacea is very abundant, especially in heather. It is about an eighth of an inch in length. D. uncinata is also often met with. Amaurobius, of which about eighty species are known, includes some species of much larger size. Three species are native to this country, A. ferox, A. similis, and A. fenestralis. A. ferox is a large and rather formidable-looking spider, more than half an inch in length, with powerful chelicerae. It is found under stones and bark, and in cellars and outhouses. A. similis is the commonest species in England, though A. fenestralis somewhat replaces it in the north. They are smaller than A. ferox, but are found in similar situations.
Fam. 18. Psechridae.—This is a small family of cribellate spiders, consisting only of two genera, Psechrus and Fecenia, and some eight species, all natives of Southern Asia and the adjacent islands. The two species of Psechrus are large spiders. They make large domed webs, which they stretch between trees or rocks, and beneath which they hang in an inverted position.
The calamistrum of these spiders is short, about half the length of the fourth metatarsus.
Fam. 19. Zodariidae (Enyoidae).—In this family are included a number of remarkable exotic spiders, most of them somewhat Drassid-like in appearance, but generally with three-clawed tarsi. The group appears to be a somewhat heterogeneous one, the twenty genera of which it consists presenting rather a wide range of characteristics.
Cydrela is an African genus of moderate sized spiders, containing five species of very curious habits. They scramble about and burrow in the sand, in which, according to Simon,[314] they appear to swim, and their chief burrowing implements are their pedipalpi, which are specially modified, the tarsi in the female bristling with spines, and being armed with one or more terminal claws.
Laches (Lachesis) includes some larger pale-coloured spiders found in Egypt and Syria, under stones in very hot and dry localities.

Fig. 206.—Hermippus loricatus, ♂ × 2½. (After Simon.)
Storena has representatives in all the tropical and sub-tropical parts of the world, and numbers about fifty species. They are of moderate size, with integuments smooth and glossy or finely shagreened, usually dark-coloured, with white or yellow spots on the abdomen. Hermippus (Fig. 206) is also African. Zodarion 400(Enyo) includes about thirty-five species of rather small, generally unicolorous spiders, very active and fond of the sunshine. They spin no web, but have a retreat under a stone. Their chief prey appear to be ants. Most of the species are native to the Mediterranean region, the others belonging to Central and Southern Asia.
Simon includes in this family the remarkable genus Cryptothele, found in Ceylon, Malacca, New Guinea, and various Oceanic islands. They are moderate sized brownish spiders, with hard integuments rugged with tubercles and projections. Their most curious characteristic is their power of retracting their spinnerets within a sort of sheath, so that they become entirely invisible.

Fig. 207.—Hersilia caudata, ♀. (After Pickard-Cambridge.)
Fam. 20. Hersiliidae.—This is a very distinct family of spiders, with broad cephalothorax, with well-marked fovea and striae, and small, well defined caput. The eyes, usually eight, are black except the median anterior pair. The legs are long and thin, and the tarsi three-clawed. The abdomen is oval or sub-globular, short haired, and generally of greyish coloration. The spinnerets supply the chief characteristic, the posterior pair being long—often excessively long—and two-jointed, the terminal joint tapering and flexible. The colulus is large. They are very active spiders, living on tree trunks or walls, or under stones, but spreading no snare. Some of them are of considerable size.
401Hersilia includes nine species native to Africa and Asia. Tama is the only genus represented in the New World, two of its species being found in South America, while others inhabit Africa, Asia, and Australia. Another genus, Hersiliola, is principally African, but extends into Spain.
Fam. 21. Pholcidae.—This is another very well-marked family. The most striking peculiarity of its members is the possession of extremely long and thin legs, the metatarsi being especially elongated, and the tarsi furnished with several false articulations.
The eyes are also very characteristic. They are usually eight in number, the two anterior median eyes being black, while the other six are white, and arranged in lateral groups of three, sometimes on prominences or stalks. The abdomen is sometimes nearly globular, but more often long and cylindrical. Most of the genera, which, including several new genera lately established by Simon, number more than twenty, are poor in species, but enjoy a very wide distribution. This is explained by the fact that many of them live in cellars and outhouses. This is the case with the genus Pholcus, of which the sole English species Ph. phalangioides is a perfect nuisance in buildings in the most southern parts of the country, “spinning large sheets of irregular webs in the corners and angles, and adding to them year by year.”[315] Other genera are Artema (Africa, South Asia, Polynesia, America), which includes the largest examples, and Spermophora, a six-eyed genus whose few species are widely distributed.
Fam. 22. Theridiidae.—Sedentary spiders, usually with feeble chelicerae and relatively large abdomen. Snare irregular.
The Theridiidae, as here understood, are a very extensive family, and more than half the British spiders (about 270 species) are included within it. This family and the next present unusual difficulties of treatment, and there is great divergence of opinion as to the most satisfactory way of dealing with them. This is chiefly due to the fact that, notwithstanding an infinite variation of facies, important points of structure are wonderfully uniform throughout both the two groups, while any differences that do occur are bridged over by intermediate forms which merge into each other.
Simon[316] has become so impressed with the difficulty of drawing 402any clear line between certain groups which he previously classed under the Theridiidae and the spiders commonly known as Epeiridae, that he has recently removed them from the Theridiidae and united them with the orb-weaving spiders to form the Family Argiopidae, the family name Epeiridae being discarded. The groups which, in his view, belong to the Argiopidae will be indicated below. This view has not met with universal acceptance, and notwithstanding the undoubted difficulty of clearly distinguishing between the two families, it is more convenient in the present work to maintain as a separate family a group of spiders nearly all of whose members possess the easily recognised characteristic of spinning a circular snare.
The Theridiidae and the Epeiridae form the great bulk of the sedentary spiders. They do not wander in search of prey, but sit in snares of various structure and wait for their victims to entangle themselves. The spinnerets, organs whose peculiarities are often strongly marked in other families, are here wonderfully constant in their arrangement and general appearance, forming a compact rosette-like group beneath the abdomen. Their eyes, normally eight in number, present an infinite variety of arrangement. Their chelicerae and mouth-parts vary considerably, but no abruptness of variation is distinguishable. This is unsatisfactory from a systematic point of view, and the necessary result is that certain groups might with equal propriety be classed with the Theridiidae or the Epeiridae. The latter family will here be taken as including all the orb-weaving spiders and a few groups which appear inseparable from them.
We shall consider the Theridiidae as comprising the seven sub-families, Argyrodinae, Episininae, Theridioninae, Phoroncidiinae, Erigoninae, Formicinae, and Linyphiinae, and shall briefly deal with them in this order.
(i.) The Argyrodinae are very curious spiders with very long and often flexible abdomen. They are commonly parasitic on the circular snares of Epeirid spiders, between the rays of which they spin their own irregular webs. There are three genera, Argyrodes, Ariamnes, and Rhomphaea, which are distributed in the tropical and sub-tropical regions all over the world.
(ii.) The Episininae hardly conform to the character of sedentary spiders, being frequently found outside their webs. In most species the abdomen is narrow in front and broader behind, 403where it is abruptly truncated or bluntly pointed. The genus Episinus is widely distributed, and one species, E. truncatus, is one of our most peculiar English spiders. It occurs occasionally under ledges of grassy or heathery banks. The genus Tomoxena is an inhabitant of tropical Asia. Janulus is found in the same regions, and in tropical America.
(iii.) The Theridioninae are a large group of spiders, often very ornate, and spinning snares of irregular threads running in all directions. The abdomen is usually more or less globular. The chelicerae are small and weak, and the paturon is transversely (not obliquely) truncated for the reception of the small unguis or fang. The somewhat long thin legs are almost or entirely destitute of spines.
We may consider certain genera as typical of the various groups into which this sub-family naturally falls. Theridion is the richest genus of the entire order, numbering some 320 species, of which seventeen inhabit the British Isles. During the summer months nearly every bush is studded with the irregular webs of these little spiders, generally prettily coloured, and with globular abdomen. The commonest is T. sisyphium, which swarms on hollies and other bushes all over the country. One of the handsomest is T. formosum, a rather local species, about a sixth of an inch in length, with the abdomen beautifully marked with oblique lines of white, yellow, red, and black. T. tepidariorum, common in conservatories, is like a large and plainer edition of T. formosum. T. riparium is remarkable for the curious earth-encrusted tube which it forms for the reception of its egg-cocoon. T. bimaculatum may often be seen among coarse herbage, holding on to its ridiculously large egg-cocoon; it is a small spider, and the sexes are more than usually unlike.
Latrodectus and Dipoena are associated exotic genera, including some of the largest species of the group. Latrodectus is peculiarly interesting on account of the great reputation for especially poisonous properties which some of its species have acquired. The New Zealand “Katipo” is L. scelio, while L. 13–guttatus enjoys an almost equally evil reputation as the “malmignatte” in Corsica. The American species L. mactans (Fig. 197, p. 362) is also considered highly venomous. These spiders form their irregular webs on low bushes, and it is curious 404that they are usually marked with red or yellow spots on the abdomen. They have been referred to in the section on the venom of spiders (see p. 362).
The genus Steatoda possesses one English species (S. bipunctata) which is extremely common in buildings and in the angles of walls, and is a rather striking spider, with dark cephalothorax, and livid brown abdomen with a broken white stripe down the middle. Several closely allied genera are also sparingly represented in this country, among which may be mentioned Crustulina (two species), Asagena (one species), Teutana (two species), Lithyphantes (one species), Laseola (five species), and Euryopis (two species). In some of these the male is provided with a stridulating organ between the thorax and abdomen (Fig. 183, p. 327). The remarkable genus Tetrablemma (see p. 318) is considered by Simon to have affinities with this group, though Pickard-Cambridge, who first described it, is inclined to rank it among the Dysderidae.

Fig. 208.—Trithena tricuspidata ♀. × 3½. (After Simon.)
(iv.) The Phoroncidiinae are a remarkable group of spiny Theridiids whose superficial resemblance to the Gasteracanthinae of the Epeiridae (see p. 409) has often deceived Arachnologists as to their true affinities. There are eight genera, all exotic, inhabiting hot countries, and spinning a Theridion-like web on bushes. Phoroncidia has twelve species in South Asia and Madagascar. Trithena (Fig. 208) is its American representative, five species being found in South America. Ulesanis has about twenty species, and extends from South America to Australia.
(v.) The Erigoninae are an immense group of minute, sober-coloured spiders, which include the “Money-spinners” of popular nomenclature, and are largely responsible for the gossamer which fills the air and covers every tuft of grass in the autumn. The number of species described is very large and constantly increasing, and more than a hundred are recognised as British.
Desperate efforts have been made of late years to grapple with this almost unmanageable group, but the multitude of genera which have been proposed can hardly as yet be considered to be finally established. The small size of these spiders, which 405renders the aid of a microscope necessary to make out their structural peculiarities, robs them of their attractiveness to any but the ardent Arachnologist, but they number among them some of our most remarkable English forms, and many of them well repay examination. The smallest English species, Panamomops diceros, measures about 1 mm. (about ¹⁄₂₅ inch) in length. Many of the groups are jet black, some with dull and others with shiny integuments. They are never greatly variegated in hue, but the glossy black of the cephalothorax, combined with red-brown or yellow legs, gives to some species a rather rich coloration.

Fig. 209.—Profile of cephalothorax of 1, Lophocarenum insanum; 2, Dactylopisthes digiticeps; 3, Walckenaera acuminata (+ abdomen); 4, Diplocephalus bicephalus; 5, Metopobractus rayi.
It is impossible here to deal with this sub-family in detail. Some of its members must be familiar enough to everybody, and the reader is recommended to spend an hour of a warm autumn day in watching them depart on the ballooning excursions, of which a description has been given (see p. 341), from the knobs which surmount iron railings in a sunny spot. Among them he is pretty sure to find the genus Erigone—containing some of the largest members of the group—strongly represented.
In some species the male presents a remarkable difference from the female in the structure of its cephalothorax, which has the head region produced into eminences sometimes of the oddest conformation. An extreme example is seen in Walckenaera acuminata, a fine species in which the male caput is produced into a sort of spire, bearing the eyes, and nearly as high as the cephalothorax is long (Fig. 209, 3).
(vi.) The Formicinae include only two genera, Formicina (South Europe) and Solenysa (Japan). They are somewhat ant-like in appearance.
(vii.) The Linyphiinae are closely allied to the Erigoninae, but the legs are usually armed with spines, and very commonly the female has a dentated claw at the end of the pedipalp.
We include here about thirty genera of spiders of moderate or small size, living for the most part on bushes or herbage. The 406characteristic Linyphian web is a horizontal sheet of irregular strands, anchored to neighbouring twigs or leaves by cross threads in all directions, and the spider generally lurks beneath the web in an inverted position. Some of the larger species are very familiar objects, Linyphia triangularis being one of the most abundant English spiders, filling furze and other bushes with its extensive spinning work.
The sub-family may be roughly divided into three groups, of which the first is small, consisting of only three exotic genera of one species each. Donachochara may be taken as the type genus. They are moderate-sized spiders with rather short legs, found in France and Holland.
The second group consists of a number of genera of small spiders, sober-coloured, and generally more or less unicolorous in brown, yellow, or black, living in herbage. The sexes are much alike, the males never exhibiting the excrescences on the caput so often met with in the Erigoninae. The genus Tmeticus may be considered the type. It includes about forty species, of which about half are British. They are mostly dull yellow or brown spiders, averaging perhaps the eighth of an inch in length. Allied genera which are represented in England are Porhomma (twelve species), Microneta (twelve species), Sintula (twelve species). The American cave-genus Anthrobia comes here.
The third and last group is that including Linyphia and allied genera. They are moderate-sized or small spiders with long spiny legs and particularly long tarsi. The abdomen is generally decorated. The caput is frequently rather prominent and crowned with hairs.
Of the large number of spiders which have been described under the generic name of Linyphia, Simon[317] only admits about fifty species. Ten are included in the British list. L. triangularis has already been mentioned, but there are other common species, as L. montana, L. marginata, and L. clathrata. The members of most of the associated genera are rather small in size. We may briefly mention Bolyphantes, Bathyphantes, Lephthyphantes, and Labulla, all of which include English species.[318]
Fam. 23. Epeiridae.—This family includes all the spiders 407which spin circular or wheel-like snares, the highest form of spider industry, together with a few forms so closely allied in structure to orb-weaving species as to be systematically inseparable from them. It is practically co-extensive with the Argiopinae, Tetragnathinae, and Nephilinae of Simon’s Argiopidae in the Histoire naturelle des araignées.[319]
No one is unfamiliar with the orbicular snares, the structure of which has already been described with some minuteness (see p. 344), and some of the spiders which construct them are among the best known members of the order.
It is impossible here to deal with the multitudinous forms embraced by this family. We must mention those genera richest in species, and some others of special interest. It will be convenient to indicate eight sub-families or groups, which include most of the forms likely to be met with. These are the Theridiosomatinae, Tetragnathinae, Argiopinae, Nephilinae, Epeirinae, Gasteracanthinae, Poltyinae, and Arcyinae.
(i.) The Theridiosomatinae are a small group which might with equal propriety be classed with the Theridiidae or the Epeiridae. Theridiosoma argenteolum is a rare spider in Dorsetshire. It is a minute spider, one-twelfth of an inch in length, with silvery white globular abdomen variegated with reddish brown, and yellow cephalothorax with darker caput. Some allied spiders spin a roughly circular snare.
(ii.) The Tetragnathinae consist chiefly of two genera, Pachygnatha and Tetragnatha. The first consists of spiders which are not orb-weavers, but live in herbage, especially in swampy places. Two species, Pachygnatha clerckii and P. degeerii, are common in England, and a third, P. listeri, is sometimes met with. They are rather striking, prettily marked spiders, with strongly developed chelicerae.
The species of Tetragnatha are true orb-weavers, and may easily be recognised by their cylindrical bodies, elongated chelicerae, and long legs, stretched fore and aft along the rays of their webs. Five species have been recorded from England, and the genus contains at least a hundred species in all; almost every country in the world, regardless of its latitude, supplying examples.
408Simon associates with these spiders the genus Meta, which includes perhaps our commonest Epeirid, Meta segmentata, a smallish and not very striking Orb-weaver, with a rather elongated or subcylindrical abdomen. Every garden is pretty sure to abound in it.
(iii.) The Argiopinae include many large and very striking members of the Epeiridae. There are about a hundred species of Argiope (Fig. 198, p. 379) spread over the tropical and sub-tropical countries of the world. They rarely invade the temperate regions, but A. bruennichi is found in South Europe, and A. trifasciata in Canada. The large spiders with transverse bars of yellow or orange on their abdomen, and often with a silvery sheen, belong to this genus. The species of the allied genus Gea are generally much smaller, and their abdomen more elongated. Both genera are found in tropical and sub-tropical regions all over the world. Argiope always sits in the middle of its circular web. There are invariably some flossy zigzag bands of silk stretched between two of the rays, and the web is generally accompanied by an irregular net on its border, where the much smaller male may be found.
(iv.) Among the Nephilinae are to be found the largest Epeirids. Indeed, the largest yield in size only to the Aviculariidae. Nephila is a tropical genus, numbering about sixty species. The abdomen is generally elongated and somewhat cylindrical, and is strikingly variegated. It is in this group that the disparity in size between the sexes is most marked (see p. 379).
(v.) The Epeirinae[320] include the bulk of the Orb-weavers, and form a very extensive group. Five genera and twenty-eight species are in the British list.
409No spider is more familiar than Epeira diademata (Fig. 181, p. 325), the Garden-spider, par excellence, which attains its greatest size and spreads its largest snares in the autumn. The smaller and much less conspicuous Zilla x-notata is sure to be found abundantly in the same locality. Several other Epeirids are to be found in this country, especially in the south, by sweeping heather or bushes with a net, or shaking the boughs of trees over an umbrella or other receptacle. The little apple-green species is Epeira cucurbitina. E. cornuta is extremely common in marshy places all over the country. In furze bushes, and often among sedge in swampy places, will frequently be found E. quadrata, one of the largest and handsomest species we possess. The ground-colour may vary from orange-red to green, and there are four conspicuous white spots on the abdomen. The tent-like retreat which this spider makes near its snare often catches the eye.

Fig. 210.—Epeira angulata, ♀.
E. umbratica is a dark flat, somewhat toad-like Epeirid of retiring habits, which stretches its snare usually on wooden palings, between the timbers of which it squeezes its flat body, and waits for insects to entangle themselves.
Two of our finest Epeiras, E. pyramidata and E. angulata (Fig. 210), are seldom met with, and only in the south.
Our only Cyclosa (C. conica) is easily recognised by the peculiar form of its abdomen, which is greatly prolonged beyond the spinnerets. It is a small, rather dark species, which constructs a particularly perfect snare.
Five British Epeirids belong to the genus Singa. They are small creatures, not exceeding a sixth of an inch in length. They live in heathery and marshy localities.
(vi.) The Gasteracanthinae are a remarkable group of Epeirids, characterised by the hard and coriaceous integument covering the abdomen, which is usually furnished with a number of more or less formidable thorn-like spines, calculated to render these spiders by no means pleasant eating for insectivorous birds. An even more constant characteristic is the presence on the back 410of the abdomen of a number of “sigilla,” or somewhat seal-like impressions arranged symmetrically, four forming a trapezium in the middle, while the others are distributed round the border.

Fig. 211.—Gasteracantha minax, ♀.
There are about 200 species of Gasteracantha, all natives of tropical countries.
The spiders of the genus Micrathena (Acrosoma) have a more elongate cephalothorax, and sometimes the spines are exceedingly long, far exceeding the length of the body proper. Among the less spiny members of this group are some remarkable mimetic ant-like forms.
(vii.) The Poltyinae include some remarkable spiders, found in Africa and South Asia for the most part, though sparingly represented in America and Oceania. They are generally largish spiders, often with a very odd conformation of the abdomen, which is generally much raised. The type genus is Poltys.
(viii.) The Arcyinae, which are more characteristic of Australia and the neighbouring islands, are a small group of spiders, usually yellow with black markings, and with the somewhat square-shaped cephalothorax usually prominent at the angles. The type genus is Arcys.
Fam. 24. Uloboridae.—The Uloboridae are cribellate spiders, with rather elongate cephalothorax, devoid of median fovea. The cribellum is transverse and generally undivided. The first pair of legs are usually much the longest. The metatarsi of the fourth legs, in addition to the calamistrum, bear a number of generally regularly arranged spines. The eyes are often situated on tubercles. Three sub-families are recognised, Dinopinae, Uloborinae, and Miagrammopinae.
(i.) The Dinopinae are a small group comprising only two genera, Dinopis and Menneus. The calamistrum is short, occupying not more than half of the metatarsus. Twenty species of Dinopis and six of Menneus are scattered over the tropical regions of the world.
(ii.) The Uloborinae include a number of spiders which have been described under several generic names, but are now considered to fall into two genera, Sybota and Uloborus. Sybota has only two 411species, one in the Mediterranean region and one in Chili. There are about sixty species of Uloborus, some of which have a wide distribution, while many (e.g. U. republicanus, of Venezuela) are social. The type species, U. walckenaerius, is a very rare spider in England.
(iii.) The Miagrammopinae include two genera containing some very interesting forms. The genus Miagrammopes, of which twenty species have been described, though the number is probably far greater, is characterised by a very long cylindrical abdomen, and by the apparent possession of only four eyes, in a transverse row. These are really the posterior eyes; and the anterior eyes, or some of them, are present in a very reduced condition. Little is known of the habits of these spiders.

Fig. 212.—Hyptiotes paradoxus, ♀.
The other genus, Hyptiotes, though only boasting three species, possesses a special interest on account of the remarkable snare constructed by the spiders which belong to it. This has already been described in the section upon defective orb-webs (see p. 349).
The type species, H. paradoxus, is very rare in England, and though small and inconspicuous, it is certainly one of the most curious members of our Spider fauna.
Fam. 25. Archeidae.—This small family includes certain remarkable fossil spiders from Baltic amber, and two rare recent forms, Archea (Eriauchenus) workmani from Madagascar, and Mecysmauchenius segmentatus from America. The chelicerae, which are extraordinarily long, are articulated far away from the mouth-parts. The caput is clearly marked off from the thorax, and is much raised. In several other respects these spiders are very distinct from all other members of the order.
Fam. 26. Mimetidae.—The Mimetidae form a small group in general appearance recalling the Theridiidae, with which family they were for a long time incorporated. The chief genera are Ero, Mimetus, and Gelanor. Ero furcata (= thoracica) is a pretty little spider, not rare among grass in England. The upper side of its very convex abdomen is marked with red, yellow, and black, and bears two little protuberances or humps near the middle. It is only about an eighth of an inch long. 412Its interesting egg-cocoon has already been alluded to (see p. 358). E. tuberculata has been found on rare occasions in this country. There are about ten other species of Ero, all small spiders, and living in temperate regions. The genus Mimetus (in which is merged Blackwall’s Ctenophora) includes a number of larger, more strongly-built spiders, living for the most part in tropical countries.
The genus Gelanor (Galena) is the American representative of the group, its three species being rather large spiders, inhabiting Central and South America. The males of this genus have remarkably long and slender pedipalpi, much longer than the whole body.
Fam. 27. Thomisidae.—The Thomisidae are the Latigrade spiders of Latreille, and the “Crab-spiders” of popular nomenclature. Their legs are extended more or less laterally instead of in the normal fore and aft directions, and their progression is frequently strikingly crab-like. They form a very large group of more than 140 genera, including spiders of every size, and they are to be found in every quarter of the world. Forty-three species are British. Many strange forms are included in this group, and several of the sub-families into which it has been divided contain only one or two genera. The bulk of its members fall into the sub-families Thomisinae, Philodrominae, and Sparassinae.
(i.) The Thomisinae (Misumeninae of Simon’s Hist. Nat.) include what may be called the more normal members of the family, distributed among more than sixty genera. Six of these genera are represented in the British Isles. Our commonest Crab-spider is probably Xysticus cristatus, abundant everywhere in grass and herbage. Young specimens may often be seen upon iron railings in the autumn. Twelve other species of that genus are on the British list. They are of small or moderate size, rarely exceeding a quarter of an inch in length. A closely allied genus is Oxyptila, of which we have seven species. The more striking members of this sub-family to be found in England are our single representatives of the genera Misumena, Diaea, and Thomisus. Misumena vatia is a handsome species, the female measuring sometimes more than a third of an inch, and having its large yellow or green abdomen marked, in many specimens, with a pair of bright red bands, which, however, are not always present. The males 413are much smaller and darker. It is common in some parts of England, especially in the south, where it is to be sought for in bushes and trees.

Fig. 213.—Thomisid spiders. A, Micrommata virescens, ♀; B, Xysticus pini, ♀; C, Philodromus margaritatus, ♂; D, Tibellus oblongus, ♀.
Diaea dorsata is one of our prettiest British species, with light green legs and cephalothorax, and a yellow abdomen with a red-brown central marking. It is common in the New Forest and other southern localities. The female attains a quarter of an inch in length.
Thomisus onustus, a rare spider among heather, is recognisable by the shape of its abdomen, which is broadest behind and abruptly truncated. When adult the abdomen is a pale yellow, but the young are suffused with a pink hue closely corresponding with that of the heather blossom in which they are frequently found sitting.
(ii.) The Philodrominae have the cephalothorax more rounded in front, and the legs, especially the second pair, usually longer than in the Thomisinae. There are ten genera, of which the most important is Philodromus, which numbers about a hundred species. They are active spiders, living upon bushes and trees, and most of them are inhabitants of temperate regions. We have about twelve species in the British Isles. The commonest is Ph. aureolus, which is abundant on bushes in most parts of the country. Some species are very prettily marked, and one, Ph. margaritatus (Fig. 213, C) presents a very good example of protective coloration, being almost indistinguishable on the blue-grey lichen on tree trunks, where it lies in wait for insects.
Another important genus, including some fifty species, is 414Thanatus, extending from tropical to arctic regions, but very sparingly represented in England. Th. striatus (= hirsutus) occurs occasionally, and one example of the fine species Th. formicinus has been taken in the New Forest. The members of this genus as a rule affect dry and sandy habitats.
The genus Tibellus includes few species, but has a wide distribution. The type species T. oblongus (Fig. 213, D) is found in the temperate regions all over the world, and is common in England. It is a pale straw-coloured spider with a much elongated abdomen. It closely resembles the stems of dry grass in hue, and when alarmed it remains perfectly still with its legs embracing the stem and its abdomen closely applied to it.
(iii.) The Sparassinae[321] include most of the large Latigrade forms, and number about forty genera.
Heteropoda venatoria is a cosmopolitan species, and though proper to warm countries, is often introduced here on hothouse plants, and has been known to establish itself in the open air in botanical gardens. Our only indigenous member of this sub-family is Micrommata virescens (Fig. 213, A). This striking spider is found, though rarely, in the south of England. The female is half an inch in length and of a vivid green hue, while the more cylindrical abdomen of the male is yellow with three longitudinal scarlet lines. Other genera are Sparassus, Torania, and Delena.
(iv.) The Aphantochilinae include two curious genera which are exclusively American. The labium is much reduced and the sternum is shortened, terminating between the third pair of legs. The species of Aphantochilus are largish, glossy-black spiders, sometimes spotted with white. Some of them mimic ants of the genus Cryptocerus. The other genus is Bucranium.
(v.) The Stephanopsinae include about sixteen genera, of which the best known are Stephanopsis and Regillus. There are about fifty species of Stephanopsis, most of them Australian, while the eight species of Regillus belong to Africa and South Asia.
The mimetic form Phrynarachne decipiens has already been alluded to (see p. 374).
(vi.) The Selenopinae consist of a single genus, Selenops, of 415which ten or twelve species are known, some of which are very widely distributed, though confined to hot regions. These spiders, which are all large, are easily recognised by their extremely flat bodies and the peculiar arrangement of their eyes, all eight of them being placed more or less in a single transverse line.
Fam. 28. Zoropsidae.—The Zoropsidae are cribellate spiders of large size, with well-developed scopulae on tarsi and metatarsi. The cribellum is divided, and the calamistrum, which is very short, is not well developed. Most are inhabitants of hot regions, where they live under stones or bark. Zoropsis has six species, chiefly inhabitants of North Africa, though representatives occur on the European side of the Mediterranean. Acanthoctenus has two species in South and Central America.
Fam. 29. Platoridae.—The Platoridae are Thomisid-like, medium-sized spiders, generally with a uniform yellow or brown coloration. The spinnerets are their most characteristic features. The median pair present a large flat surface studded with two parallel rows of large fusulae, while the anterior pair are situated outside them, and are thus widely separated. There are only three genera, and very few species of this family. Plator insolens is a Chinese species. Doliomalus and Vectius belong to South America.
Fam. 30. Agelenidae.—Sedentary spiders with slight sexual dimorphism; with three tarsal claws and devoid of scopulae.
The Agelenidae spin a more or less extensive web of fine texture, usually accompanied by a tubular retreat. Our commonest cellar spiders belong to this group, which may be divided into three sub-families, Cybaeinae, Ageleninae, and Hahniinae.
(i.) The Cybaeinae include some sixteen genera, of which two deserve special mention on account of the peculiar habits of the spiders belonging to them.
Desis is a genus of marine spiders, said to live on coral reefs below high-water mark, and to remain in holes in the rock during high tide, enclosed in cocoons impermeable to the sea-water. At low tide it is stated that they come forth and prey upon small crustaceans. Argyroneta has only one species, A. aquatica, spread throughout Europe and North and Central Asia. It is the well-known “Water-spider,” which is so often an object of interest in aquaria.
416(ii.) The Ageleninae also contain sixteen genera, but it is a much larger group, some of the genera being rich in species. They are mostly moderate or large-sized hairy spiders, living in temperate or cold climates. There are about fifty species of Tegenaria, seven of which have been recorded as British.
Our commonest Cellar-spider is T. derhamii, but the very large long-legged species found in houses in the southern counties of England is T. parietina (= guyonii = domestica). There are not many species of Agelena, but one, A. labyrinthica, is a common object in this country, with its large, close-textured web and accompanying tube spread on grassy banks by the wayside. Coelotes atropos is a formidable-looking spider, found occasionally under stones in England and Wales. Another genus, Cryphoeca, has three British representatives.
(iii.) The Hahniinae are recognised at once by their spinnerets, which are arranged in a single transverse line, the posterior pair being on the outside, and generally much the longest. Hahnia contains several species of very small spiders, of which four or five are British, usually occurring among moss or herbage. The aberrant form Nicodamus (Centropelma), usually placed among the Theridiidae, is removed by Simon to the Agelenidae, forming by itself the sub-family (iv.) Nicodaminae.
Fam. 31. Pisauridae.—The Pisauridae are hairy, long-legged spiders, intermediate, both in structure and in habits, between the Agelenidae and the Lycosidae. Many new genera have recently been added to the group, but many of them only include one or two species.
Pisaura is spread throughout the temperate regions of the Old World, and P. (Ocyale) mirabilis is common in England, being found abundantly in woods and on commons. It is a striking spider, more than half an inch in length, and its elongate abdomen is marked on either side with a sinuous longitudinal white band.
There are some thirty species of Dolomedes scattered over the temperate regions of the world. D. fimbriatus is a rare species in marshy spots in the south of England, and is one of the largest British spiders. The ground-colour is deep brown, with two longitudinal yellowish stripes both on cephalothorax and abdomen.
The genus Dolomedes is replaced by Thaumasia in South America.
417Fam. 32. Lycosidae.—These are what are popularly known as “Wolf-spiders.” They are vagabond hunting spiders, spinning no snare, but chasing their prey along the ground, and in the breeding season carrying their egg-bags with them, attached beneath the abdomen. Some of them burrow in the loose earth or sand, but others seem to have nothing in the way of a habitation.

Fig. 214.—Lycosid Spiders. 1, Lycosa fabrilis, ♀; 2, Lycosa picta, ♀; 3, Pardosa amentata, ♀.
The arrangement of the eyes is very characteristic. They are in three rows. The front row consists of four small eyes above the insertion of the chelicerae, and directed forwards. Two comparatively very large eyes form the next row, and occupy the upper angles of the facies, being also directed forwards. The third row consists of two medium-sized eyes placed dorso-laterally on the caput, some distance behind the rest, and looking upwards. The tarsi are three-clawed. The so-called “Tarantula” spiders belong to this group, though the name has been so abused in popular usage, and has passed through so many vicissitudes in scientific nomenclature, that it is difficult to tell what creature is intended by it. In America the Aviculariidae are commonly called Tarantulas.
The two chief genera of this extensive family are Lycosa and Pardosa.
The genus Lycosa includes about 400 species. It has been broken up from time to time into various genera (Trochosa, Pirata, Tarentula, etc.), but these glide into each other by imperceptible degrees, and are now discarded. They are large or moderate-sized spiders, found in every part of the world. About twenty species are British, some of them being fine and handsomely 418marked. One of the prettiest is Lycosa picta, common on the sandhills in some localities.
Some exotic species are very large, Lycosa ingens, from Madeira, measuring sometimes more than an inch and a half in length.
Pardosa (Fig. 188, p. 341) is not so rich in species, but the individuals of some species are wonderfully numerous. Hundreds of P. lugubris, for example, may be seen scampering over the dead leaves of a wood in the autumn. These spiders are generally sombrely coloured and well covered with hair. Perhaps the commonest and most widely spread species in this country is P. amentata.
Fam. 33. Ctenidae.—The Ctenidae are Lycosa-like spiders, having in certain points of structure close affinities with the Pisauridae and the Sparassinae of the Thomisidae. The limits of the family are not well defined, and many arachnologists place in it some of the genera allotted above to the Pisauridae, while others do not consider the group sufficiently marked off to constitute a separate family at all. As here understood they are equivalent to the Cteninae of the Clubionidae in Simon’s Histoire naturelle. The eyes are arranged in the Lycosa fashion, but the tarsi have only two terminal claws and well-developed “claw-tufts,” frequently accompanied by a scopula. There are strong, regularly-arranged spines under the tibiae and tarsi.
There are about fifteen genera. Uliodon numbers six species of large hairy spiders in Australia. Ctenus is rich in species, having about sixty, found in all hot countries, but especially in America and Africa. They are also of large size and usually of yellowish coloration, often diversified by a pattern on the abdomen. The fifteen species of Leptoctenus are proper to tropical Asia. Acantheis from South Asia and Enoplectenus from Brazil are more slender, elongate forms, recalling Tetragnatha. Caloctenus includes a number of Pardosa-like spiders found at a high elevation in South America.
The Ctenidae have the habits of the Lycosidae, and are wandering spiders, some forming a burrow in the ground.
Fam. 34. Senoculidae.—The South American genus Senoculus (Labdacus) alone constitutes this family. The species are probably numerous, but ten only have been described. They are moderate-sized spiders, spinning no web, but running with astonishing speed over the leaves and stems of plants. The 419generic name is really inapplicable, as there are eight eyes, but the anterior laterals are much reduced. The abdomen is long, and the legs are long and unequal, the first pair much the longest and the third much the shortest.
Fam. 35. Oxyopidae.—The Oxyopidae form a well-marked group, with oval cephalothorax somewhat narrowed in front, and lanceolate abdomen. The eight black eyes have a characteristic arrangement, and the anterior medians are always very small. The legs are long and tapering, and not very unequal, and are furnished with particularly long spines, which give these spiders a very characteristic appearance. There are eight genera, of which the most important are Pucetia and Oxyopes.
Pucetia contains a number of rather large spiders, generally bright green, often variegated with red. They affect particular plants. For instance, P. viridis, which occurs in Spain, is always found on Ononis hispanica. There are about thirty species of this genus distributed over the tropical and sub-tropical regions of the world. Oxyopes numbers many species, certainly more than fifty, and has a similar distribution, but some of its members invade colder regions. They are of rather small size. O. lineatus is a very rare spider in the south of England.
The Oxyopidae are diurnal spiders, running over plants in search of prey, and often leaping, after the fashion of members of the following family.
Fam. 36. Attidae (Salticidae).—Wandering spiders with cephalothorax broad anteriorly, and bearing eight homogeneous eyes in three rows. Four eyes, largely developed, are directed forward; the remaining four eyes are placed dorsally in two rows, the first pair being much reduced in size.
The Attidae or Jumping-spiders form the most extensive family of the whole order, the known species amounting to something like four thousand. It is only of late years that their vast numbers have begun to be realised, for their vagabond habits and great activity enabled them to a great extent to elude the earlier collectors, whose methods were not as thorough as those now in vogue. Their real home is in the tropical regions, temperate fauna being comparatively poor in Attid species. France boasts nearly 150, but only 37 are recorded for the British Isles, and 2 at least of these are recent introductions.
Some of the tropical forms are most brilliantly coloured, 420glowing with vivid colours and metallic hues, and they have frequently excited the admiration of travellers. The coloration is nearly always due to the hairs and scales with which the spiders are clothed, and is, unfortunately, almost incapable of preservation in the collector’s cabinet.
These spiders are all wanderers, spinning no snares, though they form a sort of silken cell or retreat, in which the female lays her eggs. Their habits are diurnal, and they delight in sunshine. They stalk their prey and leap upon it with wonderful accuracy. They invariably attach a thread at intervals in their course, and on the rare occasions when they miss their aim while hunting on a perpendicular surface, they are saved from a fall by the silken line proceeding from the spot whence the leap was made.

Fig. 215.—Attid Spiders. A, Salticus scenicus, ♂; B, Marpissa muscosa, ♀; C, Synemosyna formica, ♀; D, Ballus variegatus, ♀.
The movements of these spiders are sufficient to indicate their systematic position without entering upon structural details, but their eyes deserve a special mention. They are all dark-coloured and very unequal in size, and they occupy the whole area of the caput, usually forming a large quadrilateral figure. Four large eyes occupy the facies or “forehead,” the medians being especially large. Next come two very small eyes, behind the anterior laterals, and lastly two of medium size at the posterior corners of the caput.
This vast family does not lend itself easily to division into sub-families, and it will be impossible here to do more than indicate a very few of the multitudinous forms.
The most familiar British example is Salticus scenicus (Epiblemum scenicum), the little black and white striped spider to be 421seen hunting on walls and fences during the summer. Marpissa muscosa is the largest English species, measuring about half an inch. It has a brownish-yellow coloration, and is found, though not commonly, in similar situations. Attus pubescens affects grey stone walls, on which it is nearly invisible except when moving. The other British species are mostly to be found on trees and shrubs or among herbage, or hunting over bare sandy spots in the sunshine. A few (Marpissa pomatia, Hyctia nivoyi) are fen species. Hasarius falcatus is a handsome spider, common in woods in some localities.
The species differ much in their jumping powers; the Marpissas, for example, are not great leapers, but the little Attus saltator, found on sandhills, jumps like a flea, and the North American species Saitis pulex has a suggestive specific name.
Again, in this family there are mimetic forms resembling ants. Myrmarachne formicaria (Salticus formicarius) is found very rarely in England, but is not uncommon on the Continent.
Synageles and Synemosyna are allied genera. Phidippus is a genus well represented in America, and Ph. morsitans has already been mentioned (p. 365) in connexion with its poisonous reputation. Astia and Icius have American representatives (see pp. 381, 382), though the type species belongs to the Old World.
CHAPTER XVI
ARACHNIDA EMBOLOBRANCHIATA (CONTINUED)—PALPIGRADI—SOLIFUGAE = SOLPUGAE—CHERNETIDEA = PSEUDOSCORPIONES
Order IV. Palpigradi.
Minute Arachnids with three-jointed chelate chelicerae, and with the last two joints of the cephalothorax free. The abdomen consists of eleven segments with a fifteen-jointed flagellum.
In 1885 Grassi discovered, at Catania, a minute Arachnid which did not fall into any of the established orders of Arachnida. He named it Koenenia mirabilis. In 1893 Hansen collected several specimens in Calabria, near Palmi and Scilla, and carefully redescribed the species in conjunction with Sörensen.[322] It has been studied still more minutely by Börner.[323]
There is a “head” portion, covered by a carapace, and bearing the chelicerae, pedipalpi, and two pairs of legs. The two free thoracic segments bear the third and fourth pairs of legs, recalling the Schizonotidae (see p. 312), where the portion of the thorax bearing these legs is separate, though covered by a single dorsal plate. There are no eyes, but two hair-structures, believed to be sensory, are present on the cephalothorax, and Börner has observed openings in the second joint of the first pair of legs which have all the appearance of “lyriform” organs, as found in Spiders (see p. 325).
The last three abdominal segments narrow rapidly, the last bearing the anus. A fifteen-jointed caudal flagellum is carried, Scorpion-like, above the animal’s back. The body and tail are each about a millimetre in length, and the animal is of a translucent white colour.
The mouth is extremely simple, being merely a slit upon a slight eminence. There are two sternal plates beneath the “head,” and one beneath each free thoracic segment. The 423genital operculum is complicated, and is situated beneath the second abdominal segment.

Fig. 216.—Koenenia mirabilis, much enlarged. (After Hansen.)
Since 1885 several other species have been discovered in various parts of the world. Two American forms possess three pairs of lung-sacs on segments 4, 5, and 6 of the abdomen. Rucker[324] has suggested for them the generic name of Prokoenenia, including P. wheeleri, Rucker, from Texas, and P. chilensis, Hansen, from Chili. The others, styled by that author Eukoenenia, have no lung-sacs. There are about ten species, mostly from the Mediterranean region, but E. augusta, Hansen, is found in Siam, E. florenciae, Rucker, in Texas, and E. grassii, Hansen, in Paraguay.
Order V. Solifugae (Solpugae).
Tracheate Arachnids, with the last three segments of the cephalothorax free and the abdomen segmented. The chelicerae are largely developed and chelate, and the pedipalpi are leg-like, possessing terminal sense-organs.
The Solifugae are, in some respects, the most primitive of the tracheate Arachnida. Their general appearance is very spider-like, and by the old writers they are uniformly alluded to as spiders. The segmented body and the absence of spinning organs, however, make them readily distinguishable on careful inspection. They are for the most part nocturnal creatures, though some seem to rove about by day, and are even called “Sun-spiders” by the Spaniards. The night-loving species are attracted by light. They are, as a rule, exceedingly hairy. Some are extremely active, while the short-legged forms (e.g. Rhagodes, see p. 429) move slowly. They are capable of producing a hissing sound by the rubbing together of their chelicerae. Only the last three pairs of legs are true ambulatory organs, the 424first being carried aloft like the pedipalps, and used for feeling and manipulating the prey.
There has been much controversy as to the poisonous properties with which these creatures have been very widely credited by both ancient and modern writers. The people of Baku on the Caspian consider them especially poisonous after their winter sleep. The Russians of that region much dread the “Falangas,” as they call them, and keep a Falanga preserved in oil as an antidote to the bite. The Somalis, on the other hand, have no fear of them, and, though familiar with these animals, have not thought them worthy of the dignity of a name.
Several investigators have allowed themselves to be bitten without any special result. Some zoologists have found and described what they have taken to be poison-glands, but these appear to be the coxal glands, which have an excretory function. Bernard[325] suggests that, if the bite be poisonous, the virus may exude from the numerous setal pores which are found on the extremities of the chelicerae. The cutting powers of the immensely-developed chelicerae are usually sufficient to ensure fatal results on small animals without the agency of poison. Distant,[326] indeed, thinks they cannot be poisonous, for when birds attack them they flee before their assailants.
The Solifugae require a tolerably warm climate. In Europe they are only found in Spain, Greece, and Southern Russia. They abound throughout Africa, and are found in South-Western Asia, the southern United States, and the north of South America. They appear to be absent from Australia, nor have any been found in Madagascar. Their usual food appears to be insects, though they devour lizards with avidity. Some interesting observations on their habits are recorded by Captain Hutton,[327] who kept specimens in captivity in India. An imprisoned female made a burrow in the earth with which her cage was provided, and laid fifty eggs, which hatched in a fortnight, but the young remained motionless for three weeks longer, when they underwent their first moult, and became active.
A sparrow and musk rats were at different times placed in the cage, and were speedily killed, but not eaten. Two specimens placed in the same cage tried to avoid each other, but, on coming 425into contact, fought desperately, the one ultimately devouring the other. It was noteworthy that the one which was first fairly seized immediately resigned itself to its fate without a struggle. As is the case with some spiders, the female is said occasionally to kill and devour the male. A Mashonaland species, Solpuga sericea, feeds on termites,[328] while a South Californian Galeodes kills bees,[329] entering the hives in search of them. They are fairly good climbers. In Egypt Galeodes arabs climbs on to tables to catch flies, and some species have been observed to climb trees.

Fig. 217.—Rhagodes sp., ventral view. Nat. size. a, Anus; ch, chelicerae; g.o, genital operculum; n, racket organs; p, pedipalp; 1, 2, 3, 4, ambulatory legs. (After Bernard.)
That their pedipalps, in addition to their sensory function (see p. 426), possess a sucking apparatus, is clear from an observation of Lönnberg,[330] who kept specimens of Galeodes araneoides imprisoned in rectangular glass boxes, up the perpendicular sides of which they were able to climb for some distance by their palps, but, being able to obtain no hold by their legs, they soon tired.
External Anatomy.—The body of Galeodes consists of a cephalothorax and an abdomen, both portions being distinctly segmented. The cephalothorax consists of six segments, the first thoracic segment being fused with the two cephalic segments to form a sort of head, while the last three thoracic segments are free, and there is almost as much freedom of movement between the last two thoracic segments as between the thorax and the abdomen. The “cephalic lobes,” which give the appearance of a head, have been 426shown by Bernard[331] to be due to the enormous development of the chelicerae, by the muscles of which they are entirely occupied.
The floor of the cephalothorax is for the most part formed by the coxae of the appendages, and the sternum is hardly recognisable in many species. In Solpuga, however (see p. 429), it exists in the form of a long narrow plate of three segments, ending anteriorly in a lancet-shaped labium.
A pair of large simple eyes are borne on a prominence in the middle of the anterior portion of the cephalothorax, and there are often one or two pairs of vestigial lateral eyes.
The first pair of tracheal stigmata are to be found behind the coxae of the second legs.
The mouth-parts take the form of a characteristic beak, consisting of a labrum and a labium entirely fused along their sides. The mouth is at the extremity of the beak, and is furnished with a straining apparatus of complicated hairs.
The abdomen possesses ten free segments, marked off by dorsal and ventral plates, with a wide membranous lateral interval. The ventral plates are paired, the first pair forming the genital opercula, while behind the second and third are two pairs of stigmata. Some species have a single median stigma on the fourth segment, but this is in some cases permanently closed, and in the genus Rhagodes entirely absent, so that it would seem to be a disappearing structure.
The appendages are the six pairs common to all Arachnids—chelicerae, pedipalpi, and four pairs of legs. The chelicerae, which are enormously developed, are two-jointed and chelate, the distal joint being articulated beneath the produced basal joint. In the male there is nearly always present, on the basal joint, a remarkable structure of modified hairs called the “flagellum,” and believed to be sensory. It differs in the different genera, and is only absent in the Eremobatinae (see p. 429). The pedipalpi are strong, six-jointed, leg-like appendages, without terminal claw. They end in a knob-like joint, sometimes movable, sometimes fixed, which contains a very remarkable eversible sense-organ, which is probably olfactory. It is concealed by a lid-like structure, and when protruded is seen to be furnished, on its under surface, with a pile of velvet-like sensory hairs.
The legs differ in the number of their joints, as the third and 427fourth pairs have the femora divided, and the tarsus jointed. The first pair has only a very small terminal claw, but two well-developed claws are borne by the tarsi of the other legs. Each of the last legs bears, on its under surface, five “racket organs,” believed to be sensory.
Internal Structure.—The alimentary canal possesses a sucking chamber within the beak, after which it narrows to pass through the nerve-mass, and after an S-shaped fold, joins the mid-gut. This gives off four pairs of thin diverticula towards the legs, the last two entering the coxae of the third and fourth pairs.
At the constriction between the cephalothorax and the abdomen there is no true pedicle, but there is a transverse septum or “diaphragm,” through which the blood-vessel, tracheal nerves, and alimentary canal pass. The gut narrows here, and, on entering the abdomen, proceeds straight to a stercoral pocket at the hind end of the animal, but gives off, at the commencement, two long lateral diverticula, which run backwards parallel with the main trunk. These are furnished with innumerable secondary tube-like diverticula, which proceed in all directions and fill every available portion of the abdomen. The caeca, which are so characteristic of the Arachnidan mid-gut, here reach their extreme development. A pair of Malpighian tubules enter the main trunk in the fourth abdominal segment.
Other excretory organs are the coxal glands, which form many coils behind the nerve-mass, and open between the coxae of the third and fourth legs. They have been taken for poison-glands.
There is a small endosternite in the hinder portion of the cephalothorax under the alimentary canal.
The vascular system is not completely understood. The heart is a very long, narrow, dorsal tube, extending almost the entire length of the animal, and possessing eight pairs of ostia, two in the cephalothorax and six in the abdomen. It gives off an anterior and a posterior vessel, the latter apparently a vein, as it is guarded at its entrance by a valve. The blood seems to be delivered by the anterior artery on to the nerve-mass, and, after percolating the muscles and viscera, to divide into two streams—the one returning to the heart by the thoracic ostia, the other passing through the diaphragm and bathing the abdominal organs, finally to reach the heart either by the abdominal ostia or by the posterior vein.

Fig. 218.—Nervous system of Galeodes. abd.g, Abdominal ganglion; ch, cheliceral nerve; ch.f, chitinous fold; ch.r, chitinous rod; g.n, generative nerve; l, labial nerve; st, position of stigma. (After Bernard.)
The nervous system, notwithstanding the fact that the three last thoracic segments are free, is chiefly concentrated into a mass surrounding the oesophagus. Nerves are given off in front to the eyes, the labrum, and the chelicerae, while double nerves radiate to the pedipalps and to the legs. From behind the nerve-mass three nerves emerge, and pass through the diaphragm to enter the abdomen. The median nerve swells into an “abdominal ganglion” just behind the diaphragm, and is then distributed to the diverticula of the alimentary canal. The lateral nerves innervate the generative organs.
The respiratory system consists of a connected network of tracheae communicating with the exterior by the stigmata, whose position has already been described. There are two main lateral trunks extending nearly the whole length of the body, and giving off numerous ramifications, the most important of which are in the cephalothorax, and supply the muscles of the chelicerae and of the other appendages.
The generative glands do not essentially differ from the usual Arachnid type, though the paired ovaries do not fuse to form a ring. There are no external organs, and the sexes can only be distinguished by secondary characteristics, such as the “flagellum” already mentioned.
Classification.—There are about a hundred and seventy species of Solifugae inhabiting the warm regions of the earth. No member of the group is found in England, or in any except the most southern portions of Europe.
Kraepelin[332] has divided the group into three families—Galeodidae, Solpugidae, and Hexisopodidae.
Fam. 1. Galeodidae.—The Galeodidae have a lancet-shaped flagellum, directed backwards. There is a characteristic five-toothed plate or comb covering the abdominal stigmata. The tarsus of the fourth leg is three-jointed, and the terminal claws are hairy.

Fig. 219.—Chelicera and flagellum of Galeodes. (After Kraepelin.)
There are two genera, Galeodes, with about twelve species, and Paragaleodes, with six species, scattered over the hot regions of the Old World.
Fam. 2. Solpugidae.—The Solpugidae comprise twenty-four genera, distributed under five sub-families. The toothed stigmatic plate is absent, and the tarsal claws are smooth. The ocular eminence is furnished with irregular hairs. The “flagellum” is very variable.

Fig. 220.—Chelicerae and flagella of A, Rhagodes; B, Solpuga; and C, Daesia. (After Kraepelin.)
(i.) The Rhagodinae include the two genera, Rhagodes (Rhax) and Dinorhax. The first has twenty-two species, which inhabit Africa and Asia. The single species of Dinorhax belongs to East Asia. These creatures are short-legged and sluggish.
(ii.) The Solpuginae contain two genera—Solpuga with about fifty species, and Zeriana with three. They are all inhabitants of Africa, and some occur on the African shore of the Mediterranean.
(iii.) The Daesiinae number about forty species, divided among several genera, among which the principal are Daesia, Gluvia, and Gnosippus. They are found in tropical regions of both the Old and the New World.
(iv.) The Eremobatinae are North American forms, the single genus Eremobates numbering about twenty species. The flagellum is here entirely absent.

Fig. 221.—Chelicera and flagellum of Hexisopus. (After Kraepelin.)
(v.) The Karshiinae include the five genera Ceroma, Gylippus, Barrus, Eusimonia, and Karshia. They are universally distributed.
Fam. 3. Hexisopodidae.—This family is formed for the reception of a single aberrant African genus, Hexisopus, of which five species have been described.
There are no claws on the tarsus of the fourth leg, which is beset with short spine-like hairs, and in other respects the genus is peculiar.
Order VI. Chernetidea.
(CHERNETES, PSEUDOSCORPIONES.)
Tracheate Arachnids, with the abdomen united to the cephalothorax by its whole breadth. Eyeless, or with two or four simple eyes placed laterally. Abdomen segmented, with four stigmata. Chelicerae chelate, bearing the openings of the spinning organs. Pedipalpi large, six-jointed, and chelate. Sternum absent or rudimentary.
The Chernetidea or “False-scorpions” constitute the most compact and natural order of the Arachnida. There are no extreme variations within the group as at present known, while all its members differ so markedly from those of other Arachnidan orders that their true affinities are by no means easy to determine.
The superficial resemblance to Scorpions which has won these animals their popular name is almost entirely due to the comparative size and shape of their pedipalpi, but it is probable that they are structurally much more closely allied to the Solifugae.
Chernetidea are not creatures which obtrude themselves on the general notice, and it is highly probable that many readers have never seen a living specimen. This is largely due to their minute size. Garypus littoralis, a Corsican species, nearly a quarter of an inch in length of body, is a veritable giant of the tribe, while no British species boasts a length of more than one-sixth of an inch.
Moreover, their habits are retiring. They are to be sought for under stones, under the bark of trees, and among moss and débris. One species, probably cosmopolitan, certainly lives habitually in houses, and is occasionally noticed and recognised as the “book-scorpion,” and one or two other species sometimes make themselves conspicuous by the remarkable habit of seizing hold of the legs of flies and being carried about with them in their flight. With these exceptions, the Chernetidea are not likely to be seen unless specially sought for, or unless casually met with in the search for small beetles or other creatures of similar habitat. Nevertheless they are very widely distributed, and though more numerous in hot countries, are yet to be found in quite cold regions.
Though comparatively little attention has been paid to them 431in this country, twenty British species have been recorded, and the known European species number about seventy.
As might be expected from their small size and retiring habits, little is known of their mode of life. They are carnivorous, feeding apparently upon any young insects which are too feeble to withstand their attacks. The writer has on two or three occasions observed them preying upon Homopterous larvae. As a rule they are sober-coloured, their livery consisting of various shades of yellow and brown. Some species walk slowly, with their relatively enormous pedipalps extended in front and gently waving, but all can run swiftly backwards and sideways, and in some forms the motion is almost exclusively retrograde and very rapid. A certain power of leaping is said to be practised by some of the more active species. The Chernetidea possess spinning organs, opening on the movable digit of the chelicera. They do not, however, spin snares like the Spiders, nor do they anchor themselves by lines, the sole use of the spinning apparatus being, apparently, to form a silken retreat at the time of egg-laying or of hibernation.
External Structure.—The Chernetid body consists of a cephalothorax, and an abdomen composed of twelve segments. The segmentation of the abdomen is emphasised by the presence of chitinous plates dorsally and ventrally, but the last two dorsal plates and the last four ventral plates are fused, so that ordinarily only eleven segments can be counted above and nine below.
The cephalothorax presents no trace of segmentation in the Obisiinae (see p. 437), but in the other groups it is marked dorsally with one or two transverse striae. The eyes, when present, are either two or four in number, and are placed near the lateral borders of the carapace towards its anterior end. They are whitish and only very slightly convex, and are never situated on prominences. Except in Garypus there is no trace of a sternum, the coxae of the legs and pedipalps forming the ventral floor of the cephalothorax.
In the Obisiinae a little triangular projection in front of the cephalothorax is regarded by Simon[333] as an epistome. It is absent in the other sub-orders.
The abdomen is armed, dorsally and ventrally, with a series of chitinous plates with membranous intervals. The dorsal plates 432are eleven in number (except in Chiridium, which has only ten), and are frequently bisected by a median dorsal membranous line. There are nine ventral plates. There is a membranous interval down each side between the dorsal and ventral series of plates.

Fig. 222.—A, Chernes sp., diagrammatic ventral view, × about 12. a, Anus; ch, chelicera; g, generative opening; p, pedipalp; 1, 2, 3, 4, legs. (The stigmata are at the postero-lateral margins of the 1st and 2nd abdominal segments.) B, Tarsus, with claws and sucking disc.
The chitinous membrane between the plates is very extensible, rendering measurements of the body in these animals of little value. In a female full of eggs the dorsal plates may be separated by a considerable interval, while after egg-laying they may actually overlap. The four stigmata are not situated on the plates, but ventro-laterally, at the level of the hinder borders of the first and second abdominal plates.
The first ventral abdominal plate bears the genital orifice. In the same plate there are two other orifices, an anterior and a posterior, which belong to the “abdominal glands.” They were taken by some authors for the spinning organs, but their function is probably to supply material for the capsule by which the eggs are suspended from the body of the mother (see p. 434).
The Chernetidea possess chelicerae, pedipalpi, and four pairs of ambulatory legs, all articulated to the cephalothorax.

Fig. 223.—Chelicera of Garypus. f, Flagellum; g, galea; s, serrula. (After Simon.)
The chelicerae are two-jointed, the upper portion of the first joint being produced forward into a claw, curving downward. The second joint is articulated beneath the first, and curves upward to a point, the appendage being thus chelate. This second joint, or movable digit, bears, near its extremity, the opening of the spinning organ, and is furnished, at all events in the Garypinae and Cheliferinae (see p. 437), with a pectinate 433projection, the “galea,” arising at its base, and extending beyond the joint in front. In the Obisiinae it is only represented by a slight prominence.
Two other organs characterise the chelicerae of all the Chernetidea; these are the “serrula” and the “flagellum.” Their minute size and transparency make them very difficult of observation, and for a long time they escaped notice. The serrula is a comb-like structure attached to the inner side of the distal joint. The flagellum is attached to the outer side of the basal joint, and recalls the antenna of a Lamellicorn beetle, or the “pectines” of scorpions, a resemblance which gave rise to the supposition that they are olfactory organs. It is more likely, however, that they are of use in manipulating the silk.
The pedipalpi are six-jointed and are very large, giving these animals a superficial resemblance to scorpions. According to Simon,[334] the patella is absent, and the joints are coxa, trochanter, femur, tibia, tarsus, with an apophysis forming the fixed digit of the chela, while the sixth joint is the movable digit, and is articulated behind the tarsus. These joints, especially the tarsus, are often much thickened, but however strongly developed, they are always narrow and pediculate at the base. The coxae of the pedipalps are closely approximated, and are enlarged and flattened. They probably assist in mastication, but there is no true maxillary plate articulated to the coxa as in some Arachnid groups.
The legs are usually short and feeble, and the number of their articulations varies from five to eight, so that it is not easy to be certain of the homologies of the individual joints to those of other Arachnids. The coxae are large, and form the floor of the cephalothorax. They are succeeded by a short trochanter, which may be followed by another short joint, the “trochantin.” Then come the femur and tibia, elongated joints without any interposed patella, and finally the tarsus of one or two joints, terminated by two smooth curved claws, beneath which is situated a trumpet-shaped membranous sucker.
Internal Structure.[335]—The internal structure of the Chernetidea, as far as their small size has permitted it to be made out, bears a considerable resemblance to that of the Phalangidea. 434The alimentary canal dilates into a small sucking pharynx before passing through the nerve-mass into the large many-lobed stomach, but the narrow intestine which terminates the canal is convoluted or looped, and possesses a feebly-developed stercoral pocket.
Above the stomach are situated the spinning glands, the products of which pass, by seven or more tubules, to the orifice already mentioned on the distal joint of the chelicerae. The abdominal or cement-glands are in the anterior ventral portion of the abdomen. No Malpighian tubes have been found.
The tracheae from the anterior stigmata are directed forward; those from the posterior stigmata backward. Bernard[336] has found rudimentary stigmata on the remaining abdominal segments.
The heart is the usual dorsal tube, situated rather far forward, and probably possessing only one pair of ostia. The nerve-cord is a double series of ventral masses, united by transverse commissures. These undergo great concentration in the last stages of development, but in the newly-hatched Chernetid a cerebral mass and five pairs of post-oesophageal ganglia can be distinguished.
There are two peculiar eversible “ram’s-horn organs,” opening near the genital opening. They are said to be present only in the male, and have been taken for the male organs, though other writers consider them to be tracheal in function.
Development.—Some points of peculiar interest are presented by the embryology of these animals, the most striking facts being, first, that the whole of the egg is, in some cases at all events, involved in the segmentation; and, secondly, that there is a true metamorphosis, though the larva is not free-living, but remains enclosed with others in a sac attached to the mother.
At the beginning of winter the female immures herself in a silken retreat, her body distended with eggs and accumulated nourishment. About February the egg-laying commences, thirty eggs, perhaps, being extruded. They are not, however, separated from the mother, but remain enclosed in a sac attached to the genital aperture, and able, therefore, to receive the nutritive fluids which she continues to supply throughout the whole period of development.
The eggs, which line the periphery of the sac, develop into embryos which presently become larvae, that is to say, instead of further development at the expense of yolk-cells contained 435within themselves, they develop a temporary stomach and a large sucking organ, and become for a time independent sucking animals, imbibing the fluids in the common sac, and arranged around its circumference with their mouths directed towards the centre. Subsequently a second embryonic stage is entered upon, the sucking organ being discarded, and the albuminous matter which the larva has imbibed being treated anew like the original yolk of the egg.
It is an interesting fact that in this second embryonic stage a well-marked “tail” or post-abdomen is formed, and the ganglionic nerve-masses increase in number, a cerebral mass being followed by eight pairs of ganglia in the body and eight in the tail. Subsequently a great concentration takes place till, besides the cerebral mass, only five closely-applied pairs of ganglia remain, corresponding to the pedipalpi and the four pairs of legs. Moreover, the first pair advances, so as to lie on the sides of, and not behind, the oesophagus.

Fig. 224.—Three stages in the development of Chelifer. A, Segmenting ovum; B, embryo, with post-abdomen, maximum number of ganglia, and developing sucking apparatus; C, larva. (After Barrois.)
There are two ecdyses or moults during development, a partial moult, concerning only the ventral surface of the “pro-embryo” as it assumes the larval form, and a complete moult at the final stage, before emergence from the incubating sac.
At the end of winter the mother cuts a hole in the silken web, and the young brood issues forth.[337]
436Classification.—The order Chernetidea consists of a single family, Cheliferidae. Nine genera are recognised by most authors, but their grouping has been the subject of a good deal of difference of opinion, largely dependent on the different systemic value allowed by various arachnologists to the absence or presence of eyes, and to their number when present. Simon takes the extreme view that the eyes are only of specific value, and he is thus led to suppress two ordinarily accepted genera, Chernes and Roncus, which are separated chiefly by eye-characters from Chelifer and Obisium respectively. He relies rather on such characters as the presence or absence of galea, epistome, and trochantin, and establishes three sub-families as follows:—
(i.) Cheliferinae.—Galea. No epistome. Trochantin on all legs. Eyes two or none. Sole genus, Chelifer (Chelifer + Chernes).
(ii.) Garypinae.—Galea. No epistome. Trochantin on legs 3 and 4 only. Eyes four or none. Genera Chiridium, Olpium, and Garypus.
(iii.) Obisiinae.—No galea. An epistome. No trochantin. Genera Chthonius and Obisium (which includes Roncus).
Whatever be the value of the eyes in the classification of this group—and Simon adduces strong arguments for his view—there can be no doubt of their convenience in practical identification. Moreover, as Pickard-Cambridge[338] points out, a grouping of the genera according to the eyes results, as regards British species, in pretty much the same linear arrangement as Simon’s classification, and it may therefore be convenient to mention that, of the six genera represented in this country, Chthonius and Obisium are four-eyed, Roncus and Chelifer two-eyed, while Chernes and Chiridium are eyeless.
Sub-Fam. 1. Cheliferinae.—These Chernetidea have the cephalothorax slightly narrowed in front, and generally marked dorsally with two transverse striae, while the abdominal plates are bisected by a dorsal longitudinal line. With the exception of Chelifer cancroides, which is always found in houses, all the species are to be sought under bark, though occasionally they are discovered under stones.
The two genera of this sub-family are Chelifer and Chernes, the species of Chelifer being two-eyed, and those of Chernes blind.
437As already stated, Simon does not consider the possession of the two—often very feebly developed—eyes of generic importance, and admits only the genus Chelifer.
Five species of Chelifer (including Ch. cancroides) and five of Chernes have been recorded in England.

Fig. 225.—Chelifer cyrneus, enlarged. (After Simon.)

Fig. 226.—Chiridium museorum, enlarged. (After Simon.)
Sub-Fam. 2. Garypinae.—The Garypinae have the cephalothorax greatly contracted in front and often projecting considerably.

Fig. 227.—Olpium pallipes, enlarged. (After Simon.)
There are three genera, Chiridium, Olpium, and Garypus. Chiridium is eyeless, and appears to have only ten segments in the abdomen, the tergal plates of which are bisected. C. museorum is found in England, and is the only Chernetid except Chelifer cancroides which habitually lives in houses. C. ferum is found under bark in the south of France.
Neither Olpium nor Garypus, which both possess four eyes and eleven abdominal segments, have as yet been found in this country. Garypus, like Chiridium, has the dorsal abdominal plates bisected. There is a transverse stria on the cephalothorax, and the eyes are far from the anterior border. In Olpium the dorsal plates are undivided and the eyes more anterior.
Sub-Fam. 3. Obisiinae.—The cephalothorax of the Obisiinae does not narrow—and is, indeed, sometimes broadest—anteriorly. The chelicerae are notably large, and the dorsal abdominal plates undivided. They are the most active of the Chernetidea, ordinarily 438running backwards or sideways with their pedipalpi closely folded up against the body. Four genera usually admitted fall within this group:—Obisium, Roncus, Blothrus, and Chthonius.
Obisium has four eyes, parallel-sided cephalothorax, and curved chelae on the palps. Roncus is like Obisium except in having only two eyes, and is therefore disallowed by Simon, who also considers Blothrus to comprise merely eyeless species of Obisium. In Chthonius the cephalothorax is broadest in front, and the digits of the chelae are straight.
The Obisiinae are found in moss and débris, and under stones. Three species of Obisium, two of Roncus, and four of Chthonius are recorded in England.
The subjoined list of British species of Chernetidea is taken from the monograph of the Rev. O. P. Cambridge, cited above:—
CHAPTER XVII
ARACHNIDA EMBOLOBRANCHIATA (CONTINUED)—PODOGONA—PHALANGIDEA = OPILIONES—HABITS—STRUCTURE—CLASSIFICATION
Order VII. Podogona (Ricinulei).
Tracheate Arachnids with two-jointed chelate chelicerae and prehensile pedipalpi. The tarsus of the third leg of the male bears a copulatory organ.

Fig. 228.—Cryptocellus simonis, × 4. (After Hansen and Sörensen.)
In 1838 Guérin-Méneville[339] described an Arachnid from West Africa which he named Cryptostemma westermannii. At rare intervals occasional specimens of allied forms have been taken in the same region until six species of Cryptostemma have been established. In South America, also, two unique examples of very similar creatures are the only known representatives of the two species of the allied genus Cryptocellus. All the examples hitherto found are of fair size (between ⅕ inch and ½ inch in length), and bear some general, though superficial, resemblance to the Trogulidae, which has led to their being placed among the Phalangidea by almost all the Arachnologists who have noticed them. Their claim to this systematic position, however, is extremely doubtful, and Hansen and Sörensen, who have had the opportunity of studying the group much more minutely 440than previous writers, are of the opinion that they ought to constitute a separate order of Arachnids, more nearly allied to the Pedipalpi than to the Phalangidea. In this place it is only possible to indicate some of their peculiar characteristics. Their integuments are particularly hard and coriaceous. The cephalothorax is united to the abdomen by a rather broad pedicle, but there is also a remarkable coupling apparatus which makes the constriction between cephalothorax and abdomen appear very slight. There is a movable anterior projection of the cephalothorax, the “cucullus.” The two-jointed chelicerae terminate in minute chelae, as also do the five-jointed pedipalps. There are no spiracles on the abdomen, but two are situated on the thorax above the coxae of the third pair of legs. Perhaps the most remarkable fact is that, as in the Araneae, a modified limb is used by the male for the fertilisation of the female; but in this case it is not the tarsus of the pedipalp, but of the third leg of the male, which is specially developed as an intromittent organ.
Ordinal rank is not universally accorded to the group, but whatever its true position, the known forms fall under a single family Cryptostemmatidae, including the two genera Cryptostemma and Cryptocellus.
Order VIII. Phalangidea (Opiliones).
Tracheate Arachnids, with abdomen united to the cephalothorax by its whole breadth. They are oviparous, and undergo no metamorphosis. Abdomen always segmented. A pair of odoriferous glands opening on the thorax. Two simple eyes; three-jointed chelate chelicerae; pedipalpi not chelate. Spinning organs absent.
“Harvesters,” “Harvestmen,” or “Harvest-spiders,” as these animals are popularly called, need never be confounded with true Spiders if the absence of a constriction between the cephalothorax and abdomen be noted. They are more difficult to distinguish from Mites, members of which group have sometimes been described as Phalangids. The Phalangid is, however, generally recognisable by its segmented abdomen, and as a further point of distinction, it may be noted that, whereas the anal orifice is always transverse or circular in Phalangids, it is uniformly longitudinal in the Acarines.

Fig. 229.—Oligolophus spinosus. (After Pickard-Cambridge.)
Members of this group vary considerably in habit. The best known forms are exceedingly active, and trust to their speed in endeavouring to escape from danger, at the same time emitting an odorous fluid from two apertures situated just above the coxae of the first pair of legs. These active Harvestmen are only found in the mature state at certain seasons of the year, and are believed, therefore, to live only for a single season. Slow-moving forms, like the Nemastomatidae and the Trogulidae, which live amidst grass and herbage, have a much longer duration of life. In danger they remain perfectly still, and trust to their earthy appearance to escape observation.
They are stated to be extremely thirsty animals, and have been observed drinking from the dewdrops on herbage. It is probably on this account that they are sometimes seen attacking juicy vegetable matter, for without doubt they are essentially carnivorous. The larvae of insects, young spiders, mites, and myriapods are their customary food. It is not requisite that the prey should be alive, but they will not touch anything mouldy.
Notwithstanding their apparently weak mouth-parts, they do not merely suck the juices of their victims, but masticate and swallow solid particles. Cannibalism is frequently observed among them.
The males fight fiercely with one another at the breeding 442time. The females, with their long extrusible ovipositors, place groups of twenty to forty eggs in small holes in the ground or under stones or bark, unprotected by any form of cocoon. The eggs hatch into fully-formed Phalangids, which are at first white, but attain their coloration after the first moult. They subsequently moult from five to nine times.
The distribution of this group is world-wide, and some of the exotic species are very remarkable in form. Only twenty-four species have as yet been recorded in this country.
External Structure.—In the Phalangidea there is no constriction between the cephalothorax and the abdomen, and in the Ischyropsalidae alone is the distinction between them readily observable. This is due to the partial or complete fusion of the first five segments of the abdomen with the carapace or cephalothoracic shield in most species, these segments being indicated, if at all, merely by faint striae or successive transverse rows of spines or tubercles. In the forms possessing hard integuments (Gonyleptidae, Nemastomatidae, Trogulidae) this fusion results in a dorsal “scutum,” the component parts of which cannot easily be distinguished.
The cephalothorax is often surmounted by a turret—usually grooved dorsally, and beset on its edges with a spiny armature—on the sides of which are the two simple eyes. The position and shape of this turret and the arrangement of its spines are of importance in the classification of the group.

Fig. 230.—Hood of Metopoctea. (After Simon.)
In the Trogulidae the base of the turret gives rise to a remarkable, forwardly-directed, bifurcate structure, furnished with numerous strong tubular bristles. This is called the “hood,” and its hollowed-out under surface forms a chamber, the “camerostome,” in which lie the basal joints of the pedipalpi.
In most European Phalangids the under surface of the cephalothorax is almost entirely concealed by the forwardly-projecting portion of the abdomen bearing the generative opening, and by the gnathobases, not only of the pedipalpi, but of the first and sometimes of the second legs. As in Spiders, however, there is always present a “sternum” and generally a “labium.” The sternum 443is long and narrow in the Mecostethi, and Cyphophthalmi, but in the Plagiostethi, which include most of the forms found in temperate regions, it is very short and transverse, and is hidden by the abdominal prolongation before mentioned.

Fig. 231.—Mouth-parts of Phalangium. A, B, C, Gnathobases of pedipalp and first and second legs; ch, chelicera; ep, epistome; lab, labium; m, mouth; ped, pedipalp; pre.ep, pre-epistome; st, sternum, shown by the removal of the anterior part of the genital process, which extends to the dotted line; 1, 2, 3, 4, legs.
The anterior wall of the mouth is formed by a beak-like plate, the “epistome,” the basal portion of which is covered externally by a second plate, for which Simon[340] proposes the name “pre-epistome.” In some Phalangids there are three little chitinous plates, one median and two lateral, on the clypeus, between the anterior border of the carapace and the insertion of the chelicerae. They are best seen in Nemastoma.
The abdomen always presents evidences of segmentation, though there is a difference of opinion as to the number of segments of which it is composed. This is due to the already mentioned partial or complete fusion of the anterior segments with the cephalothorax. From the admirable researches of Hansen and Sörensen[341] it seems likely that the normal number of abdominal segments is ten. Ventrally, the abdomen is produced forward into a “sternal process” which is capped by a genital plate, hardly distinguishable in the Phalangidae, but readily visible in the other families, which surrounds and masks the unpaired genital orifice. Two stigmata or breathing pores are situated on the sides of the first ventral plate, which these authors consider to be composed of two fused sternites.
As in other Arachnids there are six pairs of appendages articulated to the cephalothorax. They are the chelicerae, the pedipalpi, and the four pairs of ambulatory legs.
The chelicerae are three-jointed and chelate, the second joint having its inner portion produced into an apophysis to which the final joint is apposed. In certain forms (Gonyleptidae, 444Ischyropsalis) the chelicerae are remarkably long, and may considerably exceed the total length of the trunk.
The pedipalpi are six-jointed, possessing coxa, trochanter, femur, patella, tibia, and tarsus. They are leg-like and are never chelate, but in some forms terminate in a single movable claw. The coxal joints are provided with maxillary plates.
The legs are normally seven-jointed, as in Spiders, the penultimate joint being the metatarsus. The tarsus is always multi-articulate, the number of its joints being variable. It bears terminally one or two simple claws. “False articulations” (where the parts are not inserted one into the other, but are only marked off by a membranous ring) are of frequent occurrence in the legs of these creatures. The first legs, like the pedipalps, bear maxillary plates, as do also the second in most Phalangids. The maxillae of the second legs are, however, entirely absent in Nemastoma, and rudimentary in the Gonyleptidae and the Ischyropsalidae. The coxae of the legs are all largely developed, but are not capable of free motion, being soldered to, and practically forming part of, the cephalothoracic floor. In some forms they are only separated from one another by slight grooves. The extreme length of the legs, and their hard and brittle nature, are characteristic features of the Phalangids, though in some species (Trogulidae) they are comparatively short. The first pair of legs are always the shortest, and the second the longest.
The sexual organs of Phalangids are ordinarily concealed, and the sexes can only be distinguished by certain very variable secondary characters, the males being usually smaller of body and longer of leg than the females, besides being more distinctly coloured and being armed with more numerous and longer spines. Sometimes the male chelicerae are highly characteristic.
Phalangids are usually destitute of spinning organs, but such have been discovered, in a rudimentary state, in the Cyphophthalmi, which are said to spin slight webs.
Internal Structure.—In Phalangium the mouth leads upwards into a membranous pharynx, wider than that of Spiders, but narrowing into an oesophagus which passes between the cerebral and thoracic ganglionic nerve-masses. It then turns backwards over the thoracic ganglion, being slightly dilated at 445that point. Immediately afterwards it dilates into a flask-like gastric sac which occupies almost the whole width of the abdomen, and proceeds straight to the anus. Viewed from above, the shape of this sac is entirely concealed by the large number of caeca (thirty) to which it gives rise dorsally and laterally. The two largest of these caeca extend, parallel to each other, over the whole of the abdominal portion of the gastric sac, and are flanked by four lateral pairs of smaller caeca, while there is a cluster of small caeca covering the anterior and narrower portion of the flask-like stomach.
The large hepatic mass so conspicuous on opening dorsally the abdomen of a Spider is here entirely absent, but its functions are believed to be performed by certain wrinkled, tubular, longitudinally parallel bodies, about seven in number, closely applied to the under surface of the flask.
The masticating portions of the maxillae of the pedipalpi and the first pair of legs are hollow distensible sacs, often seen in a swollen condition in specimens kept in spirits. They are furnished, on the inner surface, with a horny ridge.
Owing to the fixity of the coxae of the legs, their maxillary plates are incapable of much lateral motion, but are rubbed against each other vertically.
Beyond the fact that the heart is a dorsal tube lying along the anterior two-thirds of the alimentary canal, and divided by constrictions into three well-marked and equal portions, little is known of the blood-system of these animals. It is probably essentially like that of Spiders, but the presence of a pericardial sac has not yet been established, nor has the course of the blood-vessels been described in detail.
As in other Arachnids, the principal ganglionic nerve-masses closely embrace the oesophagus. Immediately anterior to it, forming a conical mass with its base on the oesophagus, is the cerebral ganglion, while just behind it is the transverse portion of the large thoracic nerve-centre. In Phalangium opilio, according to Tulk,[342] a median nerve is given off from the apex of the cerebral mass (the paired nature of which is apparent) and bifurcates to the two eyes. Two lateral nerves proceed to certain organs near the origin of the second pair of legs, which were thought by the old writers to be lateral eyes, but which are now 446known to be glands for the manufacture of the odorous fluid which these animals can exude.

Fig. 232.—Nervous and respiratory systems of a Phalangid. Nerves black, tracheae white. c.g, Cerebral ganglion; g′, g″, g‴, ganglia supplying viscera; m.n, median abdominal nerve; oe, passage for oesophagus; st, stigma; th.g, thoracic ganglion; tr, main trunk of tracheae.
The thoracic ganglion expands, on either side of the oesophagus, into a mass which extends nearly as far forward as the apex of the cerebral ganglion. These lateral masses give off nerves to the appendages. From the back of the transverse portion proceed three nerves. The median nerve passes above the generative organs, and soon branches into two nerves which presently swell out to form ganglia of considerable size, beyond which they soon join again and give off an anastomosing network of nerve-fibres. The lateral nerves immediately branch. The outer branch dilates into a ganglion which supplies the external part of the generative organ. The inner branch, which is longer, also forms a ganglion the nerves from which are chiefly distributed to the under surface of the alimentary canal.
The respiratory organs consist of two large tracheal tubes with numerous branches, having their external openings or “stigmata” near the base of the fourth pair of legs. The two main tubes are directed forwards, and are mainly concerned with supplying the largely developed muscles of the legs. The distribution of branches to the abdomen is comparatively feeble. The particular arrangement of tubes in P. opilio, according to Tulk, may be seen in the accompanying figure. There are a pair of coxal glands, of excretory function, opening in the neighbourhood of the coxae of the third pair of legs.
The Phalangidea are remarkable among Arachnids in the possession of large protrusible external organs of generation. The ovipositor of the female may be as long as the whole body of the animal, and the intromittent organ of the male is of 447almost equal length. The pedipalpi take no part in the fertilisation of the female, which is accomplished directly.
The protrusible organs are concealed under the forwardly-projecting anterior segment of the abdomen beneath, the genital orifice being thus in many cases quite near the head region. The internal sexual organs are not very complex. The ovary re-enters upon itself, forming a ring, and from the point of re-entry a tube proceeds towards the centre of the ring, dilating to form an ovisac. It then narrows, turns forward, dilates once more into a second ovisac, from which the oviduct proceeds to the base of the ovipositor. This is a flattened organ, grooved on its upper surface and bifid at its extremity. The testis of the male is a single sac-like gland, from either end of which proceeds a vas deferens, which, after several convolutions, unite into a sperm-sac which opens at the base of the penis.
Partial hermaphroditism is a very frequent phenomenon among the Phalangids, the testis often producing ova as well as spermatozoa.
Though the males fight fiercely at the breeding time, the animals for the most part live peacefully together. Henking[343] found that the eggs of Liobunum, which were about half a millimetre in diameter, were laid during October and hatched out in the following April.
Classification.—The Order Phalangidea is divided into three Sub-orders: 1, Cyphophthalmi; 2, Mecostethi; 3, Plagiostethi.
Sub-Order 1. Cyphophthalmi
Phalangids with dorsal and ventral scutum, only the last abdominal segment remaining free. Eyes two or absent. Maxillary lobe on coxae of first pair of legs rudimentary. Sternum long and narrow. Anterior segment of abdomen not projecting ventrally beyond the coxae of the fourth pair. Odoriferous glands open on prominences.
In 1875 Stecker published a description of a remarkable creature which he said he had found in Bohemia, and which he named Gibocellum sudeticum. Among other points it possessed four eyes and four spinning mammillae, and it differed so much from other Cyphophthalmi as to necessitate the foundation of a 448family, Gibocellidae, for its reception. No one else appears to have seen the animal, or any of Stecker’s preparations of it, and Hansen and Sörensen[344] adduce grave reasons for believing that it never existed at all. If this species is to be disallowed, the Cyphophthalmi all fall into a single family.

Fig. 233.—Parasiro corsicus, enlarged. (After Simon.)
Fam. Sironidae.—These somewhat Mite-like Phalangids are rarely met with, partly, no doubt, because of their retiring habits and small size, the known forms ranging from 6 mm. to less than 2 mm. in length. Of the seven genera which have been established, Stylocellus numbers eight species from Borneo and Sumatra, and Pettalus two species from Ceylon. Ogovia, Miopsalis, and Purcellia have one species each, from South Africa, Further India, and the Cape, respectively. The only European forms are the two species of Siro (France and Austria), and Parasiro corsicus. No species has yet been found in England.
Sub-Order 2. Mecostethi.[345]
(LANIATORES).
Sternum long and narrow. Dorsal scutum leaving at least the last three segments free. Openings of odoriferous glands not on prominences. The fourth pair of legs usually long and powerful. One terminal claw on each of the first two pairs of legs; two on the last two pairs.
The Mecostethi are essentially tropical forms, though a few representatives are found in the caves of Southern Europe. One family (Phalangodidae) has its headquarters in the hot regions of the Old World, while the other two (Cosmetidae, Gonyleptidae) are confined to Central and South America.
Fam. 1. Phalangodidae.—Body piriform or triangular, broadest behind. Last ventral segment of abdomen much the largest. Very narrow sternum. Eye-turret near anterior border of cephalothorax. Chelicerae narrow at base. Pedipalpi long and strong. Maxillary plates on first pair of legs rudimentary. No stigmata visible.
The only European forms of this family belong to the genus 449Phalangodes. They all avoid the light, and are usually found in caves. Simon[346] records six species found in France. A North American species, P. armata, is entirely destitute of eyes.

Fig. 234.—Phalangodes terricola, enlarged. (After Simon.)
The family has representatives in Australia and in tropical Africa and Asia. Mermerus, Epidanus, Maracaudus, and Sitalces are some of the exotic genera.
The other two families of this Sub-order—Fam. 2, Cosmetidae; Fam. 3, Gonyleptidae—include a large number of species, some of considerable size (up to an inch in length of body), found in Central and South America.
Sub-Order 3. Plagiostethi.[347]
(PALPATORES.)
First abdominal segment produced forward ventrally to the level of the first pair of legs, bringing the mouth and the genital opening very near together. Sternum consequently much reduced. Pedipalpi thin, with terminal claw absent or rudimentary. Terminal claws of the legs single.
The Plagiostethi include most of the Harvestmen of temperate regions, the most familiar examples of these creatures belonging to the large family Phalangidae, and being much more in evidence than the slow-moving and ground-living forms included in the other families.
Fam. 1. Phalangiidae.—Eye-turret always far removed from anterior border of cephalothorax. Second pair of legs with well-marked maxillary lobes. Legs similar, without the false joint called “trochantin.” Multiarticulate tarsi. Simple pedipalpi, with tarsus much longer than tibia, and possessing terminal claw. Some have soft, some coriaceous integuments.
The Phalangidae fall naturally into two groups or sub-families, named by Simon Sclerosomatinae and Phalangiinae. The first group consists of more or less coriaceous forms living among moss and herbage. They are not very numerous, there being only about twelve known European species divided among the three genera, Sclerosoma, Mastobunus, and Astrobunus.

Fig. 235.—Sclerosoma quadridentatum. (After Pickard-Cambridge.)
Two species of Sclerosoma are found in England, S. quadridentatum occurring not uncommonly among moss or under stones in various parts of the country. Its back is studded with wart-like tubercles, which give it a characteristic appearance.
The Phalangiinae are soft-bodied Harvestmen, always with long legs, which in the genus Liobunum attain an inordinate length. There are nine European genera, Liobunum, Prosalpia, Gyas, Oligolophus, Acantholophus, Phalangium, Dasylobus, Platybunus, and Megabunus, comprising in all about fifty species. Five of these genera are represented in England.
The familiar Phalangids, with small, almost spherical bodies and ridiculously long legs, belong to the genus Liobunum, L. rotundum being the common species. It is mature in autumn, when it may be seen scampering at a great pace among the herbage. It very readily parts with its limbs, and Pickard-Cambridge[348] relates that he once “saw one running with very fair speed and facility, having lost all but two legs, an anterior one on one side and a posterior one on the other.”
The Harvestmen so frequently seen on walls belong, as a rule, to the genus Phalangium. The best known example is Phalangium opilio (the P. cornutum of Linnaeus), the male of which possesses a remarkable development of the chelicerae.
The genus Oligolophus is well represented in this country, nine species having been recorded. They do not differ greatly from Phalangium, but have, as a rule, more massive bodies, and rather stout, though tolerably long legs. The largest English Harvestman, not rare under stones at Cambridge, is O. spinosus, whose body measures half an inch in length. O. agrestis is perhaps the commonest British Phalangid, and is abundant in woods and among herbage, and on low trees.

Fig. 236.—Oligolophus spinosus. (After Pickard-Cambridge.)
Platybunus has two, and Megabunus one British representative. They are of small size, and are to be sought for among heather or dead leaves in spring or early summer.
Fam. 2. Ischyropsalidae.—Coriaceous Phalangids, with eye-turret far removed from anterior border of cephalothorax. Maxillary lobes of second pair of legs rudimentary, in the form of tubercles. Legs similar, without “trochantin.” Multiarticulate tarsi. Tarsus of pedipalp without claw, and shorter than metatarsus. Pedipalps long and horizontal.
This family includes a small number of large or moderate-sized Phalangids, which are found occasionally in thick moss, or in caves, in mountainous regions of the south of Europe, and belong to the genera Ischyropsalis and Sabacon. There is a North American genus, Taracus.
Fam. 3. Nemastomatidae.—Coriaceous Phalangids, with cephalothorax fused with the first five segments of the abdomen, forming a scutum. Eye-turret near anterior border. No maxillary lobe on second coxae. Similar legs, without “trochantin.” Multiarticulate tarsi. Tarsus of pedipalp without claw, and shorter than metatarsus.

Fig. 237.—Nemastoma lugubre.
There is but one genus, Nemastoma, in this family, and the members of it are, as a rule, rather small and dark Phalangids, which live under stones or in moss or débris, and are found in 452the mature state at all seasons of the year. There are about twenty European species, but only two of these, N. lugubre and N. chrysomelas, have as yet been found in Britain. N. lugubre is a very common animal, and though it does not obtrude itself upon public notice, its little black body with two pearly white spots must be a familiar object to all insect collectors who have occasion to search under stones or among moss in damp places. Its legs are short and stout, but those of N. chrysomelas, which is a brighter coloured Harvestman with spots of dull gold colour, are long and slender.
Fam. 4. Trogulidae.—Coriaceous and very hard integument. Anterior part of cephalothorax produced into a bifurcate “hood.” Often a “trochantin.”

Fig. 238.—Trogulus aquaticus. a, Hood. (After Simon.)
The Trogulidae are very slow-moving Phalangids of moderate or large size (a sixth to half an inch in body), found under stones or in damp moss and débris. They are Mite-like in general appearance, and may readily be distinguished from all other Harvestmen by the presence of the “hood” (Fig. 230, p. 442), the hollowed-out under surface of which forms a chamber, called by Simon the “camerostome,” in which lie the basal portions of the pedipalps.
Only a single immature specimen has been found in England, belonging probably to the species Trogulus tricarinatus. It was found in Dorsetshire. Some members of the family are not uncommon in various regions of the Continent. There are four genera, Dicranolasma, Anelasmocephalus, Calathocratus, and Trogulus. Two other genera, Amopaum and Metopoctea, have been established, but the former is probably the young of Dicranolasma and the latter of Trogulus.
According to the monograph on the British Phalangidea by the Rev. O. Pickard-Cambridge, cited above, the following species have been recorded in this country. They all fall under the sub-order Plagiostethi:—
CHAPTER XVIII
ARACHNIDA EMBOLOBRANCHIATA (CONTINUED)—ACARINA—HARVEST-BUGS—PARASITIC MITES—TICKS—SPINNING MITES—STRUCTURE—METAMORPHOSIS—CLASSIFICATION
Order IX. Acarina (Acari, Acaridea).
Arachnids with unsegmented,[349] non-pediculated abdomen. Respiration by tracheae, or by the general surface of the body. Month parts suctorial, but frequently capable of biting or piercing. Metamorphosis always observable.
The Acarina or Mites are remarkable not so much for the number of their species, which is very considerable, as for the vast multitude of individuals of the Order, which is far in excess of that of any other Arachnid group. This fact is correlated with their minute size. Very few Mites exceed half an inch in length, while very many are microscopic creatures, often measuring less than the hundredth of an inch. Taken all round, a millimetre may be considered a large size for a Mite.
There is much variety of habit within the Order. All Mites live principally on fluid nutriment, but it may be obtained from living animals or plants or from decaying organic matter. Some are entirely parasitic upon plants or animals; others attach themselves to animals in their larval stage, but are free when adults; while others, again, live an entirely independent and predaceous life.
The greater number of the Mites are too small to strike the eye. Some of them have, however, contrived to attract attention, in no very agreeable manner. Every one knows the Mite popularly called the “Harvest-bug,” but to this day there is some uncertainty as to 455its identity. It was described as a separate species under the name of Leptus autumnalis, and Mégnin was the first to show that it was the larval form of one of the Trombidiidae (see p. 472). Most authors have considered it the larva of Trombidium holosericeum, but Murray referred it to the genus Tetranychus. The difficulty is that the minute creature cannot be removed from its victim without such injury as to prevent it from being bred out and the mature form determined. Brucker[350] has recently compared a large number of “Harvest-bugs” taken from human beings with the figures and descriptions of the larvae of certain Trombidiidae given by Henking and Berlese, and he determined them as the larvae of T. gynopterorum. Quite possibly, however, more than one genus is concerned in the production of this pest.
That certain skin-diseases are due to Mites (Demodicidae, Sarcoptidae) is a fact which is widely known. The fruit-grower, too, has to take cognisance of the Order, for his trees may suffer from “Red-spider” (Tetranychus telarius), and his black-currant bushes fail under the attack of the “Gall-mite” (Eriophyes or Phytoptus ribis). The curious swellings or galls which disfigure the leaves of many trees are sometimes of insect origin, but they are often due to Mites.
Domestic pets suffer greatly from Acarine parasites. A large number of species confine their attention exclusively to the feathers of birds (Analges, etc., see p. 466). One curious genus, Syringophilus, is parasitic within the feathers, feeding upon the pith of the quill. Heller of Kiel discovered them in 1879, but the researches of Trouessart first showed their frequent presence and very wide distribution. He found that they entered by the superior umbilicus of the feather, and disappeared by the inferior umbilicus when the feathers moulted or the infested bird died.
It is probable that the comparatively large Mites of the group Ixodoidea (see p. 468), commonly called “Ticks,” are the most widely known of the order. They attack wild and domestic animals and man, and are nearly always acquired from vegetables, such as brush or herbage. It would seem likely that many of these creatures can never have the chance of attaching themselves to animals, and it has been suggested that animal juices are a luxury but not a necessity to them, and that they can live, if 456need be, on vegetable sap, but further investigations have quite dispelled this view.
The suspected connection between the North American Tick, Boophilus annulatus, and the cattle disease known as Texas fever or “red water,” since clearly proved by the researches of Smith and Kilborne, led to the careful investigation of the life-history of that creature, and this was undertaken by Curtice.[351]
The female Ticks laid eggs a few days after dropping off the cattle, egg-laying lasting a week or more. The eggs took from three to five weeks to hatch, and the larvae attached themselves to cattle, on which they remained a fortnight, becoming mature and fertilised before they again sought the ground. The whole cycle occupied a time varying from six to ten weeks, a period apparently much exceeded by some members of the family.
Lounsbury[352] has recently made out the life-history of the South African “Bont” tick, Amblyomma hebraeum.
The eggs are deposited in the soil, ten to twenty thousand eggs in all being laid by one female. The larvae climb neighbouring plants and seize passing animals. After the third day of attachment they begin to distend, and they generally fall off, fully distended, on the sixth day, immediately seeking a place of concealment, where they become torpid. Under natural conditions the nymph does not emerge for at least eleven weeks, and then it behaves in the same way as the larva, again attaching itself to an animal for six days. A new time of torpidity and concealment ensues, again of at least eleven weeks’ duration, when the final moult takes place and the mature tick emerges. The males at once attach themselves to animals, but the females hesitate to fix themselves, except close by a male. For four days after fixation the male appears to exercise no attraction for the female, but after that period he shows great excitement at her approach. She, however, does all the courting, the male remaining fixed in the skin of the host. After pairing, the female distends greatly, attaining her maximum size (nearly one inch in length) in about a week, when she lets go and descends to the earth to lay eggs. If unmated, she detaches herself within a week, and seeks another host. Oviposition lasts from 457three to nine weeks, and the development of the egg from eleven weeks to six months. At least a year is occupied in the whole cycle. These ticks, and many others, communicate disease[353] by inoculation, conveying it from one animal to another.
No poison-glands have been demonstrated in the Acari, the function of the salivary glands of the Ticks being probably to prevent the coagulation of the blood of their victims.
It is an important point in the mode of life of the Ticks that they can live for a long time without food. Mégnin[354] states that he kept an Argas alive for four years, entirely without nutriment.
In the Tetranychinae (see p. 472), glands apparently homologous with the salivary glands of the Ticks have taken on the function of spinning organs. According to Donnadieu,[355] these glands, which resemble bunches of grapes, and are possessed by both sexes, open into the buccal cavity at the base of the chelicerae. The gummy fluid exudes from the mouth, and is combed into threads by the pedipalps. The legs of these mites are furnished terminally with curious hairs ending in a round knob, which are supposed to have some relation to their spinning habits.
The males are the busiest spinners, the time of the females being largely occupied in laying eggs among the excessively fine threads of silk with which the Mites cover the under surface of leaves. In the Eriophyidae (see p. 464) corresponding glands are thought to furnish an irritant fluid which causes abnormal growths or galls upon vegetable tissues.
External Structure.—It is often stated, but erroneously, that there is no distinction between cephalothorax and abdomen in the Mites. Certainly no such division can be made out in the Hydrachnidae (see p. 472) or in some other forms, but in the majority of Acari the cephalothorax is clearly marked off by a transverse groove or suture. In some cases the anterior portion of the cephalothorax is movably articulated with the rest, and forms a sort of false head called a “capitulum.” In most Mites the chitinous integument is soft and non-resistant, but it is otherwise with the Oribatidae or “Beetle-mites” (see 458p. 467), which are nearly all covered by an extremely hard and coriaceous armature.
Eyes are sometimes absent, sometimes present in varying numbers. They seem here to be of remarkably little systematic importance, as otherwise closely allied species may be either eyed or eyeless.
Normally Mites possess the usual Arachnid appendages, chelicerae, pedipalpi, and four pairs of ambulatory legs. The anterior appendages are, however, subject to a very great degree of modification, while in one Family, the Eriophyidae (Phytoptidae), the legs are apparently reduced to two pairs.
The chelicerae are sometimes chelate, in which case they are two-jointed, the distal joint or movable finger being always articulated below the immovable finger. Sometimes they terminate in a single claw or blade, the movable joint being obsolete. In the Ticks they exist as two long styles or piercing weapons, serrate on the outer edge.
The pedipalpi vary very much in structure, according to the habits of the particular form to which they belong. In the Sarcoptidae (see p. 466) they are hardly recognisable owing to the extent to which they have coalesced with the maxillary plate. In many of the free-living forms they are leg-like feeling organs, but in others they are raptorial, being not precisely chelate, but terminating in a “finger-and-thumb” arrangement which is of use in holding prey. The extreme development of the raptorial palp is found in Cheyletus (see p. 473), in which the whole appendage is remarkably thick and strong, and the “finger” is a powerful chitinous claw, while the “thumb” is replaced by movable pectinated spines of chitin. The Water-mites have a palpus adapted for anchoring themselves to water-weeds, the last joint being articulated terminally with the penultimate joint, and bending down upon it. Finally, in the “Snouted mites” (Bdellidae, see p. 471) the palpi are tactile or antenniform, often strongly recalling the antennae of weevils.
The maxillary plates which arise from the basal joints of the pedipalps are always more or less fused, in the Mites, to form a single median transverse plate, constituting the lower lip or “labium” of some authors. In some of the Oribatidae the fusion of the maxillae is only complete at the base, and the free points are still of some use as masticating organs. In those free 459living Mites which have undergone no great modification of the mouth-parts two other portions can be distinguished, the upper lip or “epipharynx,” and the “lingua,” which forms the floor of the mouth, and is for the most part concealed by the maxillary plate.
The legs are usually six- or seven-jointed, and are subject to great variation, especially as regards the tarsus or terminal joint. This may bear claws (1–3) or sucking disks, or a combination of the two, or may simply take the form of a long bristle or hair.
The Cheese-mite has a claw surrounded by a sucker—like Captain Cuttle’s hook within his sleeve. The claws of those species which are parasitic on the hairs of animals are sometimes most remarkably modified.

Fig. 239.—Diagram of the viscera of an Oribatid Mite, greatly enlarged. C, C, Lateral caeca of stomach; g, cerebral ganglion; od, od, oviducts; oe, oesophagus; pr.g, pro-ventricular gland; ps, pseudo-stigmatic organ; st, stomach; tr, tr, tracheae. (Partly after Michael.)
Internal Structure.—The minute size of most Mites has rendered research upon their internal structure a matter of great difficulty, and there are still many obscurities to be removed. Those forms which have been subjected to examination present a tolerable uniformity in the structure of the principal organs, but the brief description here given will not, of course, apply to aberrant groups like the Vermiformia. A marked concentration is noticeable throughout the Order, and is best exemplified by the nervous system.
The mouth leads into a sucking pharynx, which narrows to form the oesophagus. This passes through the nerve-mass in the usual Arachnid fashion, and widens to form the ventriculus or stomach. The oesophagus varies considerably in width in the various groups, being very narrow in those Mites which merely suck blood, but wider in vegetable-feeders like the Oribatidae.
The stomach is always provided with caeca, but these are not nearly so numerous as in some other Orders of Arachnida. There are always two large caeca directed backwards, and there may be others. They are most numerous in the Gamasidae (see 460p. 470), which sometimes possess eight, some being prolonged into the coxae of the legs, as in Spiders. At the sides of the anterior part of the stomach there are usually two glandular bodies, the pro-ventricular glands. In those Mites in which the alimentary canal is most differentiated (e.g. Oribatidae) three parts are distinguishable behind the stomach, a small intestine, a colon, and a rectum, but in most groups the small intestine is practically absent. The Malpighian tubes, very variable in length, enter at the constriction between colon and rectum.
In some of the Trombidiidae there appears to be a doubt as to the existence of a hind-gut at all. A body having the appearance of the hind-gut, and occupying its usual position, is found to contain, not faecal matter, but a white excretory substance, and all efforts to discover any passage into it from the stomach have been unsuccessful. Both Croneberg[356] and Henking[357] came to the conclusion that the stomach ended blindly, and that the apparent hind-gut was an excretory organ. Michael,[358] in his research upon a Water-mite, Thyas petrophilus, met with precisely the same difficulty, and was led to the belief that what was originally hind-gut had become principally or entirely an excretory organ.
The nervous system chiefly consists of a central ganglionic mass, usually transversely oval, and presenting little or no indication of the parts which have coalesced in its formation. Nerves proceed from it in a radiate manner, but no double nerve-cord passes towards the posterior end of the body. As above stated, it is perforated by the oesophagus.
The vascular system is little understood. In 1876 Kramer[359] wrote that he was able to perceive an actively pulsating heart in the posterior third of the abdomen in specimens of Gamasus which had recently moulted, and were therefore moderately transparent. No other investigator has been equally fortunate, though many capable workers have sought diligently for any trace of a dorsal vessel in various Acarine groups.
It would appear that the blood-flow in most Mites is lacunar and indefinite, aided incidentally by the movements of the muscles, and perhaps by a certain rhythmic motion of the 461alimentary canal, which has been observed to be most marked during the more quiescent stages of the life-history.
The internal reproductive organs have the ringed arrangement generally observed in the Arachnida. The two testes, which are sometimes bi-lobed, are connected by a median structure which may serve as a vesicula seminalis, and there are two vasa deferentia which proceed to the intromittent organ, which is sometimes situated quite in the anterior part of the ventral surface, but at others towards its centre. The male Mite is often provided with a pair of suckers towards the posterior end of the abdomen, and sometimes accessory clasping organs are present.
In some Mites there is no intromittent organ, and Michael[360] has described some remarkable cases in which the chelicerae are used in the fertilisation of the female, a spermatophore, or bag containing spermatozoa, being removed by them from the male opening and deposited in that of the female. The most remarkable instance is that of Gamasus terribilis, the movable joint of whose chelicera is perforated by a foramen through which the spermatophore is, so to speak, blown and carried as a bi-lobed bag, united by the narrow stalk which passes through the foramen, to the female aperture.
The ovaries are fused in the middle line, and connected by oviducts with the tube (vagina or uterus) which passes to the exterior. There is often an ovipositor.
Professor Gené of Turin[361] described, in 1844, some remarkable phenomena in connection with the reproduction of Ticks. The male Ixodes introduced his rostrum into the female aperture, two small white fusiform bodies emerging right and left from the labium at the moment of introduction. On retraction they had disappeared. When the female laid eggs, a bi-lobed vesicle was protruded from beneath the anterior border of the scutum and grasped the egg delivered to it by the ovipositor, appearing to manipulate it for some minutes. Then the vesicle was withdrawn, and the egg was left on the rostrum, and deposited by it in front of the animal. When the vesicle was punctured, and so rendered useless, the unmanipulated eggs quickly shrivelled and dried up.
Lounsbury[362] has recently confirmed Professor Gené’s observation 462as to oviposition in the case of a South African Tick, Amblyomma hebraeum.
The respiratory organs, if present, are always in the form of tracheae. These are usually long and convoluted, but not branching. The spiral structure is difficult to make out in these animals, and in the Oribatidae at least, instead of the external sheath being fortified with a spiral filament of chitin, there is a very delicate enveloping membrane with an apparently unbroken chitinous lining, which can, however, by suitable treatment, be resolved into a ribbon-like spiral band.[363] The position of the stigmata is very variable, and is utilised to indicate the main groups into which the Mites have been divided.
The Oribatidae possess two curious cephalothoracic organs which were for a long time considered respiratory. These are in the form of two bodies, like modified hairs, which protrude from sockets on the dorsal surface of the cephalothoracic shield. Michael[364] has shown that these have no connection with the tracheae, and he regards them as sensory organs—possibly olfactory. They are generally referred to as the “pseudo-stigmatic” organs.
In the Oribatidae, at all events, well-developed coxal glands are present. In many Mites, especially the Ixodoidea or Ticks, the salivary glands are large and conspicuous.
Metamorphosis.—All Mites undergo a metamorphosis, varying in completeness in the different groups. Altogether six stages can be recognised, though they are seldom or never all exhibited in the development of a single species. These are ovum, deutovum, larva, nymph, hypopial stage, and imago.
The Ovum.—All Mites lay eggs. It is frequently stated that the Oribatidae are viviparous exceptions, but though some of them are perhaps ovoviviparous, most deposit eggs like the rest of the Order. A phenomenon which has probably helped to foster this erroneous view is the occasional emergence from the dead body of the mother of fully-formed larvae. Towards winter it is not unusual for the mother to die at a time when her abdomen contains a few ripe eggs, and these are able to complete their development internally.
The Deutovum.[365]—In a few cases (Atax, Damaeus) a stage has 463been observed in which the outer envelope of the egg becomes brown and hard, and splits longitudinally, so as to allow the thin inner membrane to become visible through the fissure. More room is thus obtained for the developing larva, which is, moreover, protected, over most of its surface, by a hard shell. The deutovum stage may occur either within the body of the mother, or after the egg has been laid.
The Larva.—Omitting, for the moment, the very aberrant Vermiformia (see p. 464), it is the almost universal rule for the egg to hatch out as a hexapod larva. The larvae of the genus Pteroptus are said to be eight-legged. Winkler has stated that the early embryo of Gamasus possesses eight legs, of which the last pair subsequently atrophy, but this observation requires confirmation.
The Nymph.—The nymph-stage commences on the acquisition of eight legs, and lasts until the final ecdysis which produces the imago. This is the most important period of Acarine life, and is divided into a prolonged active period, during which the animal feeds and grows, and an inert period, sometimes prolonged, but at others very short, and differing little from the quiescence observable at an ordinary moult, during which the imago is elaborated. In many species the nymph is strikingly different from the imago; in others there is a close resemblance between them. It would appear, from the cases which have been most thoroughly investigated, that the imago is not developed, part for part, from the nymph, but that there is an “histolysis” and “histogenesis” similar to that which occurs among certain insects (see vol. v. p. 165). There may be more than one nymphal stage.
The hypopial stage occurs in the Tyroglyphinae, the “Cheese-mite” sub-family. Here some of the young nymphs assume an entirely different form, so different that it was for a long time considered to constitute a separate genus, and was named Hypopus. The animal acquires a hard dorsal covering. The mouth-parts are in the form of a flat blade with two terminal bristles, but with no discernible orifice. The legs are single-clawed, and all more or less directed forward, and they are articulated near the middle line of the ventral surface. Suckers are always present under the hind part of the abdomen.
It appears that these remarkably modified nymphs are entrusted with the wider distribution of the species, and that 464they are analogous to the winged individuals which occur in the parthenogenetic generations of the Aphidae. The ordinary Tyroglyphus is soft-bodied, and requires a moist environment, and exposure to the sun or prolonged passage through the air would be fatal to it. The hypopial form is much more independent of external conditions, and its habit is to attach itself by its suckers to various insects, and by this means to seek a new locality, when it resumes the ordinary nymph-form and proceeds with its development.
Classification.—There is no generally accepted classification of the Acarina, though several eminent Arachnologists have attempted of late years to reduce the group to order. Widely different views are held concerning the affinities of certain groups, and there is no agreement as to the value to be accorded to the characters which all recognise. Thus Canestrini[366] allows thirty-four families, while according to Trouessart[367] there are only ten.
Trouessart’s scheme of classification is in the main followed in the present chapter.
Sub-Order 1. Vermiformia.
This Sub-order includes the lowest and most aberrant forms of the Mites. They are entirely parasitic, and of very small size. The abdomen is much elongated, and is transversely striated. There are two families, Eriophyidae[368] (Phytoptidae) and Demodicidae.
Fam. 1. Eriophyidae (Phytoptidae).—These are the so-called Gall-mites. The curious excrescences and abnormal growths which occur on the leaves and buds of plants are familiar to every one. Various creatures are responsible for these deformities, many being the work of insects, especially the Cynipidae among the Hymenoptera, and the Cecidomyiidae among the Diptera. Others, again, are due to Eriophyid Mites.
Though the galls originated by Mites are often outwardly extremely similar to those of insect origin, they can be at once distinguished on close examination. Mite-galls contain a single chamber, communicating with the exterior by a pore, usually 465guarded with hairs, and the Mites live gregariously within it, apparently feeding upon the hairs which grow abundantly on its inner surface. In Insect-galls each insect larva lives in a separate closed chamber.

Fig. 240.—Vermiform Mites, highly magnified. A, Demodex folliculorum; B, Eriophyes (Phyptoptus) ribis.
The Eriophyidae are unique among Mites in possessing only two pairs of legs, situated quite at the anterior part of the body. The mouth-parts are very simple.
There are three genera, Eriophyes (Phyptoptus) with about one hundred and fifty known species, Monochetus with a single species, and Phyllocoptes with about fifty species.
Among the best known examples are Eriophyes tiliae, which produces the “nail-galls” on lime-leaves, and E. ribis, the “black-currant Gall-mite,” which feeds between the folded leaves of the leaf-buds, and gives rise to swelling and distortion.
Fam. 2. Demodicidae.—The single genus Demodex which constitutes this family consists of a few species of microscopic Mites which inhabit the hair-follicles of mammals, and are the cause of what is known as “follicular mange,” some other forms of mange being due to members of the succeeding family. Demodex possesses eight short, three-jointed legs, each terminated by two claws. The abdomen is much produced, and is transversely striated. About ten species have been described, but of these five are probably varieties of D. folliculorum (Fig. 240, A), which infests Man.
Sub-Order 2. Astigmata.
The Astigmata are Mites of more or less globular form, with chelate chelicerae and five-jointed legs. All members of the group are eyeless. Their habits are very various, some feeding on vegetable matter and others on carrion, while a large number are parasitic on animals. Tracheae are absent. There is only one family.

Fig. 241.—A, Leg of a fowl infested with “leg-scab”; B, female of Sarcoptes mutans, greatly magnified. (After Neumann.)
Fam. 1. Sarcoptidae.—No tracheae or stigmata. Apical rostrum. Oviparous or ovoviviparous. The seventy genera and 530 odd species of this family are divided into a number of sub-families, of which the principal are the Sarcoptinae, the Analgesinae, and the Tyroglyphinae.
(i.) The Sarcoptinae are the so-called “Itch-mites.” They are minute animals, with bodies transversely wrinkled and legs terminating in suckers or bristles. The genus Sarcoptes, which includes about fifteen species, lives in tunnels which it burrows in the skin of mammals.
(ii.) The Analgesinae are the “Birds’-feather Mites.” The principal genera are Pterolichus (120 species), Pteronyssus (33 species), Analges (23 species), Megninia (42 species), and Alloptes (33 species).
(iii.) The Tyroglyphinae[369] have received the popular name of “Cheese-mites,” from the best known example of the group. They are smooth-bodied, soft-skinned white Mites, with legs usually terminating in a single claw, sometimes accompanied by a sucker. They are for the most part carrion-feeders, living upon decaying animal or vegetable matter, but a few are parasitic on mammals, insects, and worms.
There are sixteen genera, including about fifty species. Tyroglyphus siro and T. longior are common Cheese-mites. Other species live in decaying vegetables and food-stuffs. Some of the genus Glycyphagus (G. palmifer, G. plumiger) are very remarkable for the palmate or plumose hairs which decorate their bodies. The remarkable hypopial stage in the development of Tyroglyphus has been mentioned on p. 463. The Tyroglyphinae are the lowest of the free-living Acarine forms.
Sub-Order 3. Metastigmata.
The four families which constitute this sub-order comprise a large number of Mites in which the tracheae open near the articulation of the legs, and consequently in a somewhat posterior situation. The families are Oribatidae, Argasidae, Ixodidae, and Gamasidae.
Fam. 1. Oribatidae.—The Oribatidae or “Beetle-mites” are free-living Acari, with tracheae of which the stigmata are concealed by the articulation of the legs. The cephalothorax is distinctly marked off from the abdomen, and bears dorsally two “pseudo-stigmatic” organs. The rostrum is inserted below the cephalothorax. These Mites gain their popular name from the beetle-like hardness of their integuments. They are oviparous or ovoviviparous. Eyes are always absent.

Fig. 242.—Oribatid Mites. A, Cepheus ocellatus, × 24; B, ventral view of Hoploderma magnum, closed, × 20. (After Michael.)
These are small creatures, seldom attaining the twentieth of an inch in length. They are vegetable-feeders (except, perhaps, Pelops), and are to be found in dead wood or vegetable débris, under bark, or among moss and lichen. In winter they often take refuge under stones. It is impossible at present to estimate the number of existing species, for only a few localities have been systematically worked for them, and their small size has prevented their inclusion, in any numbers, in the collections of scientific expeditions. Our knowledge of the group is likely, however, to be largely extended, for it has been found that they reach England alive and in good condition from the most remote regions if moss or other material in which they live is collected when not too dry, and hermetically sealed up in tin cases.
About twenty genera and more than 220 species are at present known. Pelops has much elongated chelicerae, with very small chelae at the end. There are ten species, found in moss and on bushes. Oribata numbers about fifty species, found in moss and on trees. Notaspis, in which the 468last three legs are inserted at the margin of the body, has about thirty species, found among moss and dead leaves. Nothrus is a short-legged genus with flat or concave dorsal plate, often produced into very remarkable spiny processes. There are twenty-two species found under bark and among moss and lichen. Hoploderma (Hoplophora) is remarkable for its power of shutting down its rostrum and withdrawing its legs in a manner which leaves it as unassailable as a tortoise or an armadillo.
Though the Oribatidae are all eyeless, they are distinctly sensitive to light, not wandering aimlessly till they reach a shadow, but apparently making straight for a dark spot when subjected to strong illumination. Some species have a curious habit of collecting dirt and débris on their backs, so as entirely to obscure the often very remarkable disposition of the spines and processes with which they are furnished.

Fig. 243.—Capitulum of Boophilus australis; ventral view. p1, p2, p3, p4, The four articles of the palp; m, the mandible or chelicera; d, its digit; n, the hypostome.
The next two families include the animals commonly known as Ticks, the largest and most familiar of the Mite tribe. Of recent years they have attracted much attention as the conveyers, to man and domestic animals, of certain diseases due to blood-parasites (see p. 457, n.), and our knowledge of their structure and habits has greatly increased in consequence. Hitherto they have generally been considered to constitute a single family, the Ixodidae, but a section of them so differ from the rest as to require their removal to another family, the Argasidae, so that it is necessary to employ a super-family name—Ixodoidea—to embrace the whole group.
Ticks are parasitic on mammals, birds, and reptiles, some shewing a marked partiality for a particular host, others being much more catholic in their tastes. Both sexes in the Argasidae, but the females only of the Ixodidae, are capable of great distension, but when unfed they are all somewhat flat animals with laterally extended legs and rather crab-like movement.
All Ticks possess a small, movable “false head” or capitulum bearing mouth-parts which are exceedingly characteristic of the 469group. The chelicerae are cutting instruments with their distal ends serrated outwardly, and there is always present a hypostome beset with recurved teeth which serve to maintain a firm hold on the tissues into which it is thrust. On either side of the chelicerae are the four-jointed palps, leg-like in the Argasidae, but more rigid and rod-like in the Ixodidae, where their inner margin is often hollowed so as to enclose the chelicerae and hypostome when the palps are apposed. There is a conspicuous pair of spiracles near the coxae of the fourth pair of legs.

Fig. 244.—Ornithodoros talaje, under surface, × 5. (After Canestrini.)
Fam. 2. Argasidae.—The Argasidae are leathery Ticks without a shield or scutum, and with free, leg-like palps. The capitulum is never more than partially visible when the adult animal is viewed dorsally. Their hosts are always warm-blooded animals. Two genera are usually recognised, Argas and Ornithodoros, though recent discoveries of new forms have tended towards their fusion. Argas reflexus and A. persicus have been proved to convey a Spirochaete disease to fowls, and the latter, under the name of the “Mianeh Bug” has long possessed an evil reputation for the “poisonous” effect of its bite on human beings. In Mexico the “Turicata” (Ornithodoros turicata) and the “Garapata” (O. megnini) are greatly dreaded, while human “tick fever” on the Congo has been traced to the instrumentality of O. moubata.

Fig. 245.—Female Sheep-tick, Ixodes ricinus.
Fam. 3. Ixodidae.—These are the more familiar Ticks, possessing a scutum or shield, which covers the whole back of the male, which is capable, therefore, of little distension, whereas it forms only a small patch on the front part of the body of the distended female. There are ten genera, Ixodes, Haemaphysalis, Dermacentor, Rhipicentor, Rhipicephalus, Boophilus, Margaropus, Hyalomma, Amblyomma, and Aponomma.
Ixodes ricinus is the common English sheep-tick. Species 470of Boophilus are parasitic on cattle the world over, and B. annulatus is the transmitter of Texas fever. Rhipicephalus and Amblyomma are large genera which include several species of economic importance. For example, R. sanguineus conveys canine piroplasmosis, and A. hebraeum causes “heart-water” in South African cattle. The genus Aponomma confines its attention to reptiles, and some of its species are exceedingly ornate.
Neglecting Margaropus and Rhipicentor, which include only a very few aberrant forms, the following entirely artificial key will serve to differentiate the genera of the Ixodidae:—
| 1. | A pair of eyes on the lateral borders of the scutum | 2 |
| No eyes | 6 | |
| 2. | Capitulum long, much longer than broad | 3 |
| Capitulum short | 4 | |
| 3. | Unicolorous, ♂ with chitinous plates near anus | Hyalomma |
| Generally ornate, ♂ without anal plates | Amblyomma | |
| 4. | Generally ornate, ♂ without anal plates, but with enlarged 4th coxae | Dermacentor |
| Unicolorous, ♂ with anal plates and normal coxae | 5 | |
| 5. | Palpi very short, spiracle circular | Boophilus |
| Palpi medium, spiracle comma-shaped | Rhipicephalus | |
| 6. | Capitulum short; 2nd article of palp projecting laterally | Haemaphysalis |
| Capitulum long | 7 | |
| 7. | Unicolorous, elongate, on birds or mammals | Ixodes |
| Generally ornate, broad-oval, on reptiles | Aponomma |
Neumann has recently revised the Ixodoidea in a series of papers published in the Mémoires de la Société zoologique de France,[370] but the work is not obtainable as a whole. A monograph, by Nuttall, Warburton, Cooper, and Robinson, is now in course of publication at the Cambridge University Press.[371]
Fam. 4. Gamasidae.—The Gamasidae are carnivorous Mites, either free-living or parasitic on animals. The chelicerae are chelate, and the palps are free. The tarsi have two claws, accompanied by a “caruncle” or sucking disc. They are mostly pale-coloured Mites, with a smooth, more or less scutate covering. The three principal sub-families are Gamasinae, Uropodinae, and Dermanyssinae.
Of the Gamasinae, Gamasus coleoptratorum is the well-known Beetle-parasite so frequently seen on Geotrupes. It is often confounded with another species of similar habits, G. crassipes.
471The curious Beetle-parasites attached to their victim by a thread belong to the genus Uropoda of the Uropodinae. The connecting filament, which the Mite can sever at will, for a long time puzzled observers. It was variously construed as a silken cord of attachment, and as a sort of umbilical cord, through which the Mite drew nourishment from the Beetle. On more careful investigation it proved to be connected with the anus of the Mite, and to consist of its consolidated excrement.
The Dermanyssinae are all parasitic on warm-blooded animals, principally birds and bats. Dermanyssus avium is the common parasite infesting fowls and cage-birds.
Sub-Order 4. Heterostigmata.
Fam. Tarsonemidae.—This is the sole family of the sub-order. It comprises a number of minute vegetable-feeding Mites which have been little studied, though they are probably the cause of considerable injury to the leaves and buds of plants.
Sub-Order 5. Prostigmata.
In these Mites the stigmata are situated anteriorly, in the rostrum or the thorax. In the Water-mites the tracheae have atrophied, but these creatures are clearly Trombidiidae which have taken to an aquatic life.

Fig. 246.—Bdella lignicola, x about 50. (After Canestrini.)
Fam. 1. Bdellidae.—The Bdellidae are sometimes known as the “Snouted Mites” on account of the very prominent forwardly-directed “capitulum” or false head. They have chelate chelicerae and tactile palps, which are often “elbowed,” like the antennae of weevils. Eyes may be present or absent. They are usually of a bright red colour, and are free-living and predaceous, though in their larval stages they may often be found attached to the limbs of insects and spiders.
The minute active scarlet Mites of the genus Eupodes and its allies perhaps come within this family. Their legs are six-jointed.
472The remaining families of the Prostigmata (Halacaridae, Hydrachnidae, and Trombidiidae) all have raptorial palps, and clawed or piercing chelicerae.
Fam. 2. Halacaridae.—This is a small group of marine Mites. In their usually prominent capitulum they resemble the Bdellidae. In some respects they recall the Oribatidae, having hard integuments, and their legs being articulated near the margin of the body. They do not swim, but crawl upon weeds and zoophytes, or burrow in the mud.

Fig. 247.—Atax alticola, x 16. (After Canestrini.)
Fam. 3. Hydrachnidae.—The Hydrachnidae are the Fresh-water Mites. Their legs are provided with long close-set hairs, and thus adapted for swimming. They are predaceous, and in their young stages are often parasitic on water insects. A familiar example is Atax bonzi, which lives within the shell of the fresh-water mussel.
Fam. 4. Trombidiidae.—The predaceous palps of the Trombidiidae are generally of the “finger-and-thumb” type. The tarsi are two-clawed, without caruncle. This group may be divided into six sub-families.

Fig. 248.—Tetranychus gibbosus, x 50. (After Canestrini.)
(i.) The Limnocharinae or “Mud-mites” connect the Hydrachnidae with the typical Trombidiidae. They are usually velvety and of a red colour. They do not swim, but creep. The larva of Limnocharis aquaticus is parasitic on Gerris lacustris.
(ii.) The Caeculinae bear a strong general resemblance to the Harvestmen or Phalangidae. Caeculus is so similar to the Phalangid genus Trogulus that it was considered by Dufour to belong to the same order.
(iii.) The Tetranychinae or “Spinning mites” are phytophagous, and do much injury to plants, sucking the sap from the leaves and giving them a blistered appearance. Tetranychus telarius is the “Red-spider” of popular nomenclature.
473(iv.) The Cheyletinae are remarkable Mites with fleshy, semi-transparent body, and enormously developed raptorial pedipalpi, which are extremely formidable weapons of attack. They do not creep or run like most Mites but proceed by a series of short leaps. Cheyletus is the principal genus.
The curious genus Syringophilus, which is parasitic in the interior of birds’ feathers, appears to be a degenerate Cheyletine.
(v.) The Erythraeinae are minute, active Mites, usually red in colour, free-living and predaceous.
(vi.) The Trombidiinae include most of the moderate-sized, velvety red Mites which are commonly known as “Harvest-mites,” and their larvae, the so-called Harvest-bugs, frequently attack man. Trombidium holosericeum is a well-known example.
Sub-Order 6. Notostigmata.[372]
This sub-order has been established for the reception of the curious genus Opilioacarus.
Fam. Opilioacaridae.—Mites with segmented abdomen, leg-like palps, chelate chelicerae, and two pairs of eyes. There are four dorsal abdominal stigmata. Four species of the sole genus Opilioacarus have been recorded, O. segmentatus from Algeria, O. italicus from Italy, O. arabicus from Arabia, and O. platensis[373] from South America.
CHAPTER XIX
TARDIGRADA
OCCURRENCE—ECDYSIS—STRUCTURE—DEVELOPMENT—AFFINITIES—BIOLOGY—DESICCATION—PARASITES—SYSTEMATIC
The animals dealt with in this chapter lead obscure lives, remote from the world, and few but the specialist have any first-hand acquaintance with them. Structurally they are thought to show affinities with the Arachnida, but their connexion with this Phylum is at best a remote one.
Tardigrades are amongst the most minute multicellular animals which exist, and their small size—averaging from ⅓ to 1 mm. in length—and retiring habits render them very inconspicuous, so that as a rule they are overlooked; yet Max Schultze[374] asserts that without any doubt they are the most widely distributed of all segmented animals. They are found amongst moss, etc., growing in gutters, on roofs, trees or in ditches, and in such numbers that Schultze states that almost any piece of moss the size of a pea will, if closely examined, yield some members of this group, but they are very difficult to see. The genus Macrobiotus especially affects the roots of moss growing on stones and old walls. M. macronyx lives entirely in fresh water, and Lydella dujardini and Echiniscoides sigismundi are marine; all other species are practically terrestrial, though inhabiting very damp places.
In searching amongst the heather of the Scotch moors for the ova and embryos of the Nematodes which infest the alimentary canal of the grouse, I have recently adopted a method not, as far as I am aware, in use before, and one which in every 478case has yielded a good supply of Tardigrades otherwise so difficult to find. The method is to soak the heather in water for some hours and then thoroughly shake it, or to shake it gently in a rocking machine for some hours. The sediment is allowed to settle, and is then removed with a pipette and placed in a centrifugaliser. A few turns of the handle are sufficient to concentrate at the bottom of the test-tubes a perfectly amazing amount of cryptozoic animal life, and amongst other forms I have never failed to find Tardigrades.

Fig. 249.—Dorsal view of Echiniscus testudo, C. Sch., × 200, showing the four segments 1, 2, 3, 4. (From Doyère.)

Fig. 250.—Cast-off cuticle of Macrobiotus tetradactylus, Gr., × about 150, containing four eggs in which the boring apparatus of the embryo can be distinguished. (From R. Greeff.)
Many Tardigrades are very transparent; their cells are large, and arranged in a beautifully symmetrical manner; and since those of them that live in moss, and at times undergo desiccation, are readily thrown into a perfectly motionless state, during which they may be examined at leisure, it is not surprising that these little creatures have been a favourite object 479for histological research. One way to produce the above-mentioned stillness is partly to asphyxiate the animals by placing them in water which has been boiled, and covering the surface of the water with a film of oil.

Fig. 251.—Echiniscus spinulosus, C. Sch., × about 200, seen from the side. (From Doyère.)
The whole body is enclosed in a thin transparent cuticle, which must be pierced by a needle if it be desired to stain the tissues of the interior. As a rule the cuticle is of the same thickness all over the body, but in the genus Echiniscus the cuticle of the dorsal surface is arranged in thickened plates, and these plates are finely granulated. From time to time the cuticle is cast, and this is a lengthy process, so that it is not unusual to find a Tardigrade ensheathed in two cuticles, the outer of which is being rubbed off. The Macrobioti lay their eggs in their cast cuticle (Fig. 250). The end of each of the eight legs bears forked claws of cuticular origin. The legs are not jointed except in the genus Lydella, where two divisions are apparent.
Within the cuticle is the epidermis, a single layer of cells arranged in regular longitudinal and transverse rows along the upper and under surface, where the cells are as uniformly arranged and as rectangular as bricks. The cells on the sides of the body are polygonal, and not in such definite rows. The nuclei show the same diagrammatic symmetry as the cells which contain them, and lie in the same relative position in neighbouring cells. In a few places, such as the end of each limb and around the mouth and arms, the cells of the epidermis are heaped up and form a clump or ridge. In some genera a deposit of pigment in the epidermis, which increases as the animal grows old, obscures the internal structures. It is generally brown, black, or red in colour.

Fig. 252.—Macrobiotus schultzei, Gr., × 150. (Modified from Greeff.) a, The six inner papillae of the mouth; b, the chitin-lined oesophagus; c, calcareous spicule; d, muscle which moves the spicule; e, muscular pharynx with masticating plates; f, salivary glands; g, stomach; h, ovary; i, median dorsal accessory gland; k, diverticula of rectum.
The cuticle and epidermis enclose a space in which the various internal organs lie. This space is traversed by numerous symmetrically disposed muscle-fibres, and contains a clear fluid—the blood—which everywhere bathes these organs. This fluid 480evaporates when desiccation takes place, and is soon replaced after rain; it forms no coagulum when reagents are added to it, and it probably differs but little from water. Floating in it are numerous corpuscles, whose number increases with age. In well-fed Tardigrades the corpuscles are packed with food-reserves, often of the same colour—green or brown—as the contents of the stomach, which soon disappear when the little creatures are starved.
The alimentary canal begins with an oral cavity, which is in many species surrounded by chitinous rings. The number of these rings and their general arrangement are of systematic importance. The oral cavity opens behind into a fine tube lined with chitin, very characteristic of the Tardigrada, which has been termed the mouth-tube. By its side, converging anteriorly, lie the two chitinous teeth, which may open ventrally into the mouth-tube, as in Macrobiotus hufelandi and Doyeria simplex, or may open directly into the oral cavity, as in Echiniscus, Milnesium, and some species of Macrobiotus. In some of the last named the tips of the teeth are hardened by a calcareous deposit. The hinder end of each stylet or tooth is 481supported by a second chitinous tooth-bearer,[375] and the movement of each is controlled by three muscles, one of which, running forwards to the mouth, helps to protrude the tooth, whilst the other two running upwards and downwards to the sheath of the pharynx, direct in what plane the tooth shall be moved.
The mouth-tube passes suddenly into the muscular sucking pharynx, which is pierced by a continuation of its chitinous tube. Roughly speaking, the pharynx is spherical; the great thickness of its walls is due to radially arranged muscles which run from the chitinous tube to a surrounding membrane. When the muscles contract, the lumen of the tube is enlarged, and food, for the most part liquid, is sucked in. Two large glands, composed of cells with conspicuous nuclei, but with ill-defined cell outlines, pour their contents into the mouth in close proximity to the exit of the teeth. The secretion of the glands—often termed salivary glands—is said in many cases to be poisonous.
The pharynx may be followed by a distinct oesophagus, or it may pass almost immediately into the stomach, which consists of a layer of six-sided cells arranged in very definite rows. In fully-fed specimens these cells project into the lumen with a well-rounded contour. Posteriorly the stomach contracts and passes into the narrow rectum, which receives anteriorly the products of the excretory canals and the reproductive organs, and thus forms a cloaca. Its transversely placed orifice lies between the last pair of legs. The food of Tardigrades is mainly the sap of mosses and other humble plants, the cell-walls of which are pierced by the teeth of the little creatures.
The organs to which an excretory function has been attributed are a pair of lateral caeca, which vary much in size according as the possessor is well or ill nourished. They recall the Malpighian tubules of such Mites as Tyroglyphus. Nothing comparable in structure to nephridia or to coxal glands has been found.
The muscles show a beautiful symmetry. There are ventral, dorsal, and lateral bundles, and others that move the limbs and teeth, but the reader must be referred to the works of Basse, Doyère,[376] and Plate[377] for the details of their arrangement. The muscle-fibres are smooth.

Fig. 253.—Brain of Macrobiotus hufelandi, C. Sch., × about 350. (From Plate.) Seen from the side. ap, Lobe of brain bearing the eye; ce, supra-oesophageal ganglion; d, tooth; Ga, first ventral ganglion; ga’, sub-oesophageal ganglion; k, thickening of the epidermis round the mouth; oc, eye-spot; oe, oesophagus; op, nerve running from the ocular lobe of the brain to the first ventral ganglion; ph, pharynx.
The nervous system consists of a brain or supra-oesophageal ganglion, whose structure was first elucidated by Plate, and a ventral chain of four ganglia. Anteriorly the brain is rounded, and gives off a nerve to the skin; posteriorly each half divides into two lobes, an inner and an outer. The latter bears the eye-spot when this is present. Just below this eye a slender nerve passes straight to the first ventral ganglion. The brain is continued round the oral cavity as a thick nerve-ring, the ventral part of which forms the sub-oesophageal ganglion, united by two longitudinal commissures to the first ventral ganglion. Thus the brain has two channels of communication between it and the ventral nerve-cord on each side, one by means of the slender nerve above mentioned, and one through the sub-oesophageal ganglion. The ventral chain is composed of four ganglia connected together by widely divaricated commissures. Each ganglion gives off three pairs of nerves, two to the ventral musculature, and one to the dorsal. The terminations of these nerves in the muscles are very clearly seen in these transparent little creatures, though there is still much dispute as to their exact nature.
The older writers considered the Tardigrada as hermaphrodites, but Plate and others have conclusively shown that they are bisexual, at any rate in the genus Macrobiotus. The males are, however, much rarer than the females. The reproductive organs of both sexes are alike. Both ovary and testis are unpaired structures opening into the intestine, and each is provided with a dorsal accessory gland placed near its orifice. In the ovary many of the eggs are not destined to be fertilised, but serve as nourishment for the more successful ova which survive.
No special circulatory or respiratory organs exist, and, as in many other simple organisms, there is no connective tissue.

Fig. 254.—Male reproductive organs of Macrobiotus hufelandi, C. Sch., × about 350. (From Plate.) a.ep, Epidermal thickening round anus; cl, cloaca; gl.d, accessory gland; gl.l, Malpighian gland; st, stomach; te, testis; x, mother-cells of spermatozoa.
The segmentation of the egg in M. macronyx is total and equal, according to the observations of von Erlanger.[378] A blastula, followed by a gastrula, is formed. The blastopore closes, but later the anus appears at the same spot. There are four pairs of mesodermic diverticula which give rise to the coelom and the chief muscles. The reproductive organs arise as an unpaired diverticulum of the alimentary canal, which also gives origin to the Malpighian tubules. The development is thus very primitive and simple, and affords no evidence of degeneration.
With regard to their position in the animal kingdom, writers on the Tardigrada are by no means agreed. O. F. Müller placed them with the Mites; Schultze and Ehrenberg near the Crustacea; Dujardin and Doyère with the Rotifers near the Annelids; and von Graff with the Myzostomidae and the Pentastomida. Plate regards them as the lowest of all air-breathing Arthropods, but he carefully guards himself against the view that they retain the structure of the original Tracheates from which later forms have been derived. He looks upon Tardigrades as a side twig of the great Tracheate branch, but a twig which arises nearer the base of the branch than any other existing forms. These animals seem certainly to belong to the Arthropod phylum, inasmuch as they are segmented, have feet ending in claws, Malpighian tubules, and an entire absence of cilia. The second and third of these features indicate a relationship with the Tracheate groups; on the other hand there is an absence of paired sensory appendages, and of mouth-parts. Von Erlanger has pointed out that the Malpighian tubules, arising as they do from the mid-gut, are not homologous with the Malpighian tubules of most Tracheates, 484and he is inclined to place this group at the base or near the base of the whole Arthropod phylum. They, however, show little resemblance to any of the more primitive Crustacea. The matter must remain to a large extent a matter of opinion, but there can be no doubt that the Tardigrades show more marked affinities to the Arthropods than to any other group of the animal kingdom.
Biology.—Spallanzani, who published in the year 1776 his Opuscules de physique animale et végétale, was the first satisfactorily to describe the phenomena of the desiccation of Tardigrades, though the subject of the desiccation of Rotifers, Nematodes, and Infusoria had attracted much notice, since Leeuwenhoek had first drawn attention to it at the very beginning of the century. In its natural state and in a damp atmosphere Tardigrades live and move and have their being like other animals, but if the surroundings dry up, or if one be isolated on a microscopic slide and slowly allowed to dry, its movements cease, its body shrinks, its skin becomes wrinkled, and at length it takes on the appearance of a much weathered grain of sand in which no parts are distinguishable. In this state, in which it may remain for years, its only vital action must be respiration, and this must be reduced to a minimum. When water is added it slowly revives, the body swells, fills out, the legs project, and gradually it assumes its former plump appearance. For a time it remains still, and is then in a very favourable condition for observation, but soon it begins to move and resumes its ordinary life which has been so curiously interrupted.
All Tardigrades have not this peculiar power of revivification—anabiosis, Preyer calls it—it is confined to those species which live amongst moss, and the process of desiccation must be slow and, according to Lance,[379] the animal must be protected as much as possible from direct contact with the air.
According to Plate, the Tardigrada are free from parasitic Metazoa, which indeed could hardly find room in their minute bodies. They are, however, freely attacked by Bacteria and other lowly vegetable organisms, and these seem to flourish in the blood without apparently producing any deleterious effects on the host. Plate also records the occurrence of certain enigmatical spherical bodies which were found in the blood or more usually in the cells 485of the stomach. These bodies generally appeared when the Tardigrades were kept in the same unchanged water for some weeks. Nothing certain is known as to their nature or origin.
Systematic.—A good deal of work has recently been done by Mr. James Murray on the Polar Tardigrades and on the Tardigrades of Scotland, many of which have been collected by the staff of the Lake Survey.[380] Over forty species have been described from North Britain.
The following table of Classification is based on that drawn up by Plate:—
I. The claws of the legs are simple, without a second hook. If there are several on the same foot they are alike in structure and size.
A. The legs are short and broad, each with at least two claws.
B. The legs are long and slender; each bears only one small claw.
II. The claws of the legs are all or partly two- or three-hooked. Frequently they are of different lengths.
A. There are no processes or palps around the mouth.
I. The muscular sucking pharynx follows closely on the mouth-tube.
α. The oral armature consists on each side of a stout tooth and a transversely placed support.
β. The oral armature consists on each side of a stylet-like tooth without support.
II. The mouth-tube is separated from the muscular sucking pharynx by a short oesophagus.
B. Six short processes or palps surround the mouth, and two others are placed a little farther back.
1. Genus ECHINISCUS (= EURYDIUM, Doy.).—The dorsal cuticle is thick, and divided into a varying number of shields, which bear thread- or spike-like projections. The anterior end forms a proboscis-like extension of the body. Two red eye-spots. There are many species, and the number has increased so rapidly in the last few years that specialists are talking of splitting up the 486genus. E. arctomys, Ehrb.; E. mutabilis, Murray; E. islandicus, Richters; E. gladiator, Murray; E. wendti, Richters; E. reticulatus, Murray; E. oihonnae, Richters; E. granulatus, Doy.; E. spitzbergensis, Scourfield;[381] E. quadrispinosus, Richters; and E. muscicola, Plate, are all British. More than one-half of these species are also Arctic, and E. arctomys is in addition Antarctic. In fact, the group is a very cosmopolitan one. The genus is also widely distributed vertically, specimens being found in cities on the sea level and on mountains up to a height of over 11,000 feet.

Fig. 255.—Diphascon chilenense, Plate, × about 100. (From Plate.) ce, Brain; k, thickening of the epidermis above the mouth; o, egg; oe, oesophagus; p,?salivary glands; ph, pharynx; sa, blood corpuscles; st, stomach.
1a. Sub-genus ECHINISCOIDES differs from the preceding in the number of the claws, the want of definition in the dorsal plates, and in being marine. The single species E. sigismundi, M. Sch., is found amongst algae in the North Sea (Ostend and Heligoland).
2. Genus LYDELLA.[382]—The long, thin legs of this genus have two segments, and in other respects approach the Arthropod limb. Marine. Plate suggests the name L. dujardini for the single species known.
3. Genus MACROBIOTUS has a pigmented epidermis, but eye-spots may be present or absent. The eggs are laid one at a time, or many leave the body at once. They are either quite free or enclosed in a cast-off cuticle. The genus is divided into many species and shows signs of disruption. They mostly live amongst moss; but M. macronyx, Doy., is said to live in fresh water. The following species are recorded from North Britain: M. oberhäuseri, Doy.; M. hufelandi, Schultze; M. zetlandicus, Murray; M. intermedius, Plate; M. angusti, Murray; M. annulatus, Murray; 487M. tuberculatus, Plate; M. sattleri, Richters; M. papillifer, Murray; M. coronifer, Richters; M. crenulatus, Richters; M. harmsworthi, Murray; M. orcadensis, Murray; M. islandicus, Richters; M. dispar, Murray; M. ambiguus, Murray; M. pullari, Murray; M. hastatus, Murray; M. dubius, Murray; M. echinogenitus, Richters; M. ornatus, Richters; M. macronyx?, Doy.
4. Genus DOYERIA.—The teeth of this genus have no support, and the large salivary glands of the foregoing genus are absent; in other respects Doyeria, with the single species Doyeria simplex, Plate, resembles Macrobiotus, and is usually to be found in consort with M. hufelandi, C. Sch.
5. Genus DIPHASCON resembles M. oberhäuseri, Doy., but an oesophagus separates the mouth-tube from the sucking pharynx, and the oral armature is weak. The following species are British, the first named being very cosmopolitan, being found at both Poles, in Chili, Europe, and Asia: D. chilenense, Plate; D. scoticum, Murray; D. bullatum, Murray; D. angustatum, Murray; D. oculatum, Murray; D. alpinum, Murray; D. spitzbergense, Murray.
6. Genus MILNESIUM has a soft oral armature, and the teeth open straight into the mouth. A lens can usually be distinguished in the eyes. Two species have been described, M. tardigradum, Doy., British, and M. alpigenum, Ehrb. Bruce and Richters consider that these two species are identical.
CHAPTER XX
PENTASTOMIDA[383]
OCCURRENCE—ECONOMIC IMPORTANCE—STRUCTURE—DEVELOPMENT AND LIFE-HISTORY—SYSTEMATIC
Pentastomids are unpleasant-looking, fluke-like or worm-like animals, which pass their adult lives in the nasal cavities, frontal sinuses, and lungs of flesh-eating animals, such as the Carnivora, Crocodiles, and Snakes; more rarely in Lizards, Birds, or Fishes. From these retreats their eggs or larvae are sneezed out or coughed up, or in some other way expelled from the body of their primary host, and then if they are eaten, as they may well be if they fall on grass, by some vegetable-feeding or omnivorous animal, they undergo a further development. If uneaten the eggs die. When once in the stomach of the second host, the egg-shell is dissolved and a larva emerges (Fig. 260, p. 494), which bores through the stomach-wall and comes to rest in a cyst in some of the neighbouring viscera. Here, with occasional wanderings which may prove fatal to the host, it matures, and should the second host be eaten by one of the first, the encysted form escapes, makes its way to the nasal chambers or lungs, and attaching itself by means of its two pairs of hooks, comes to rest on some surface capable of affording nutriment. Having once taken up its position the female seldom moves, but the males, 489which are smaller than the females, are more active. They move about in search of a mate. Further, should the host die, both sexes, after the manner of parasites, attempt to leave the body. Like most animals who live entirely in the dark they develop no pigment, and have a whitish, blanched appearance.
The only species of Pentastomid which has any economic importance is Linguatula taenioides of Lamarck, which is found in the nose of the dog, and much more rarely in the same position in the horse, mule, goat, sheep, and man. It is a comparatively rare parasite, but occurred in about 10 per cent of the 630 dogs in which it was sought at the laboratory of Alfort, near Paris, and in 5 out of 60 dogs examined at Toulouse. The symptoms caused by the presence of these parasites are not usually very severe, though cases have been recorded where they have caused asphyxia. The larval stages occur in the rabbit, sheep, ox, deer, guinea-pig, hare, rat, horse, camel, and man, and by their wandering through the tissues may set up peritonitis and other troubles.
As in the Cestoda, which they so closely resemble in their life-history, the nomenclature of the Pentastomids has been complicated by their double life. For long the larval form of L. taenioides was known by different names in different hosts, e.g. Pentastoma denticulatum, Rud., when found in the goat, P. serratum, Fröhlich, when found in the hare, P. emarginatum when found in the guinea-pig, and so on. In the systematic section of this article some of the species mentioned are known in the adult state, some in the larval, and in only a few has the life-history been fully worked out.
Structure.[384]—The body of a Pentastomid is usually white, though in the living condition it may be tinged red by the colour of the blood upon which it lives. The anterior end, which bears the mouth and the hooks (Fig. 256), has no rings; this has been termed the cephalothorax. The rest of the body, sometimes called the abdomen, is ringed, and each annulus is divided into an anterior half dotted with the pores of certain epidermal glands and a hinder part of the ring in which these are absent.
On the ventral surface of the cephalothorax, in the middle 490line, lies the mouth, elevated on an oral papilla, and on each side of the mouth are a pair of hooks whose bases are sunk in pits. The hooks can be protruded from the pits, and serve as organs of attachment. Their shape has some systematic value.

Fig. 256.—Porocephalus annulatus, Baird. A, Ventral view of head, × 6; B, ventral view of animal, × 2.
There are a pair of peculiar papillae which bear the openings of the “hook-glands,” lying just in front of the pairs of hooks, and other smaller papillae are arranged in pairs on the cephalothorax and anterior annuli. The entire body is covered by a cuticle which is tucked in at the several orifices. This is secreted by a continuous layer of ectoderm cells. Some of these subcuticular cells are aggregated together to form very definite glands opening through the cuticle by pores which have somewhat unfortunately received the name of stigmata. Spencer attributes to these glands a general excretory function. There is, however, a very special pair of glands, the hook-glands, which extend almost from one end to the other of the body; anteriorly these two lateral glands unite and form the head-gland (Fig. 257). From this on each side three ducts pass, one of which opens to the surface on the primary papilla; the other two ducts open at the base of the two hooks which lie on each side of the mouth. Leuckart has suggested that these important glands secrete some fluid like the irritating saliva of a Mosquito which induces an increased flow of blood to the place where it is of use to the parasite. Spencer, however, regards the secretion as having, like the secretion of the so-called salivary cells of the Leech, a retarding action on the coagulation of the blood of the host.
The muscles of Pentastomids are striated. There is a circular layer within the subcuticular cells, and within this a longitudinal 491layer and an oblique layer which runs across the body-cavity from the dorso-lateral surface to the mid-ventral line, a primitive arrangement which recalls the similar division of the body-cavity into three chambers in Peripatus and in many Chaetopods. Besides these there are certain muscles which move the hooks and other structures.
The mouth opens into a pharynx which runs upwards and then backwards to open into the oesophagus (Fig. 257). Certain muscles attached to these parts enlarge their cavities, and thus give rise to a sucking action by whose force the blood of the host is taken into the alimentary canal. The oesophagus opens by a funnel-shaped valve into the capacious stomach or mid-gut, which stretches through the body to end in a short rectum or hind-gut. The anus is terminal.

Fig. 257.—Diagrammatic representation of the alimentary, secretory, nervous, and reproductive systems of a male Porocephalus teretiusculus, seen from the side. The nerves are represented by solid black lines. (From W. Baldwin Spencer.)
1, Head-gland; 2, testis; 3, hook-gland; 4, hind-gut; 5, mid-gut; 6, ejaculatory
duct; 7, vesicula seminalis; 8, vas deferens; 9, dilator-rod sac; 10, cirrus-bulb;
11, cirrus-sac; 12, fore-gut; 13, oral papillae.
There appears to be no trace of circulatory or respiratory organs, whilst the function usually exercised by the nephridia or Malpighian tubules or by coxal glands, of removing waste nitrogenous matter, seems, according to Spencer, to be transferred to the skin-glands.
The nervous system is aggregated into a large ventral ganglion which lies behind the oesophagus. It gives off a narrow band devoid of ganglion-cells, which encircles that tube. It also gives off eight nerves supplying various parts, and is continued backward as a ninth pair of prolongations which, running along the ventral surface, reach almost to the end of the body (Fig. 257). The only sense-organs known are certain paired papillae on the head, which is the portion that most closely comes in contact with the tissues of the host.
492Pentastomids are bisexual. The males are as a rule much less numerous and considerably smaller than the females, although the number of annuli may be greater.
The ovary consists of a single tube closed behind. This is supported by a median mesentery. Anteriorly the ovary passes into a right and left oviduct, which, traversing the large hook-gland, encircle the alimentary canal and the two posterior nerves (Fig. 258). They then unite, and at their point of union they receive the ducts of the two spermathecae, usually found packed with spermatozoa. Having received the orifices of the spermatheca, the united oviducts are continued backward as the uterus, a highly-coiled tube in which the fertilised eggs are stored. These are very numerous; Leuckart estimated that a single female may contain half a million eggs. The uterus opens to the exterior in the mid-ventral line a short distance—in P. teretiusculus on the last ring but seven—in front of the terminal anus. In L. taenioides the eggs begin to be laid in the mucus of the nose some six months after the parasite has taken up its position.

Fig. 258.—Diagrammatic representation of the alimentary, secretory, nervous, and reproductive systems of a female Porocephalus teretiusculus, seen from the side. The nerves are represented by solid black lines. (From W. Baldwin Spencer.)
1, Head-gland; 2, oviduct; 3, hook-gland; 4, mid-gut; 5, ovary; 6, hind-gut; 7,
vagina; 8, uterus; 9, accessory gland; 10, spermatheca.
The testis is a single tube occupying in the male a position similar to that of the ovary in the female. Anteriorly it opens into two vesiculae seminales, which, like the oviducts, pierce the hook-glands and encircle the alimentary canal (Fig. 257). Each vesicula passes into a vas deferens with a cuticular lining. Each vas deferens also receives the orifice of a muscular caecal ejaculatory duct, which, crowded with mature spermatozoa, stretches back through the body. Anteriorly the vas deferens passes into a cirrus-bulb, which is joined by a cirrus-sac on one side and a dilator-rod sac on the other, structures containing 493organs that assist in introducing the spermatozoa into the female. The two tubes then unite, and having received a dorsally-placed accessory gland, open to the exterior by a median aperture placed ventrally a little way behind the mouth.
Life-history.—The egg undergoes a large portion of its development within the body of the mother. In Linguatula taenioides, which lives in the nasal cavities of the dog, the eggs pass away with the nasal excretions. If these, scattered about in the grass, etc., be eaten by a rabbit, the egg-shell is dissolved in the stomach of the second host and a small larva is set free. In Porocephalus proboscideus and others, which inhabit the lungs of snakes, the eggs pass along the alimentary canal and leave the body with the faeces. They also must be eaten by a second host if development is to proceed.

Fig. 259.—A late larval stage of Porocephalus proboscideus, seen from the side. Highly magnified. (From Stiles.) 1, primordium of first pair of chitinous processes; 2, primordium of second pair of chitinous processes; 3, mouth; 4, ventral ganglion; 5, receptaculum seminis; 6, oviduct; 7, ovary; 8, anus; 9, vagina.
The larva which emerges when the egg-shell is dissolved has a rounded body provided with two pairs of hooked appendages, and a tail which is more or less prominent in different species (Figs. 259, 260). Each appendage bears a claw, and is strengthened by a supporting rod or skeleton. Anteriorly the head bears a boring apparatus of several chitinous stylets. The various internal organs are in this stage already formed, though in a somewhat rudimentary state, and it is doubtful if the anus has yet appeared.

Fig. 260.—Larva of Porocephalus proboscideus, seen from below. Highly magnified. (From Stiles.) 1, Boring, anterior end; 2, first pair of chitinous processes seen between the forks of the second pair; 3, ventral nerve-ganglion; 4, alimentary canal; 5, mouth; 6 and 7, gland-cells.
By means of its boring apparatus, and aided by its hooked limbs, the larva now works its way through the stomach-walls of its second host, and comes to rest in the liver or in some other viscus. Its presence in the tissues of its second host causes the formation of a cyst, and within this the larva rests and develops. In man, at least, the cysts often undergo a 494calcareous degeneration, and Virchow states “dass beim Menschen das Pentastomum am häufigsten von allen Entozoen zu Verwechselungen mit echten Tuberkeln Veranlassungen giebt.” The larva moults several times, and loses its limbs, which seem to have no connexion with the paired hooks in the adult (Fig. 256). The internal organs slowly assume the form they possess in the adult. The larva is at first quite smooth, but as it grows the annulations make their appearance, arising in the middle and spreading forward and backward (Fig. 259). In this encysted condition the larva remains coiled up for some months, according to Leuckart; six in the case of L. taenioides, and a somewhat shorter period, according to Stiles,[385] in the case of P. proboscideus.

Fig. 261.—Encysted form of Porocephalus protelis, × 1, lying in the mesentery of its host. (From Hoyle.)
The frequency of what used to be called Pentastoma denticulatum (= the larval form of L. taenioides) in the body of man depends on the familiarity of man with dogs. Klebs and Zaeslin found one larva in 900 and two in 1914 autopsies. Laenger[386] found the larva fifteen times in about 400 dissections, once in the mesentery, seven times in the liver, and seven times in the wall of the intestine. After remaining encysted for some time it may 495escape, and begins wandering through the tissues, aided by its hooks and annulations, a proceeding not unaccompanied by danger to its host. Should the latter be eaten by some carnivorous animal, the larva makes its way into the nasal cavities or sinuses, or into the lungs of the flesh-eating creature, and there after another ecdysis it becomes adult. If, however, the second host escapes this fate, the larvae re-encyst themselves, and then if swallowed they are said to bore through the intestine of the flesh-eater, and so make their way to their adult abode.
Systematic.[387]—The Pentastomida are a group much modified by parasitism, which has so deeply moulded their structure as to obscure to a great extent their origin and affinities. The larva, with its clawed limbs, recalls the Tardigrades and certain Mites, e.g. Phytoptus, where only two pairs of limbs persist, and where the abdomen is elongated and forms a large proportion of the body. The resemblances to a single and somewhat aberrant genus must not, however, be pressed too far. The striated muscles, the ring-like nature of the reproductive organs and their ducts, perhaps even the disproportion both in size and number of the females to the males, are also characters common to many Arachnids.
The Pentastomida include three genera, Linguatula, Fröhlich, Porocephalus, Humboldt, and Reighardia, Ward.[388] The first two were regarded by Leuckart as but sub-genera, but Railliet[389] and Hoyle[390] have raised them to the rank of genera. They are characterised as follows:—
Linguatula, body flattened, but dorsal surface arched; the edges of the fluke-like body crenelated; the body-cavity extends as diverticula into the edges of the body.
Porocephalus, body cylindrical, with no diverticula of the body-cavity.
Reighardia, devoid of annulations, transparent, with poorly developed hooks and a mouth-armature.
496The following is a list of the species with their primary and secondary or larval hosts:—
i. Linguatula pusilla, Diesing, found in the intestine of the fresh-water fish Acara, a South American genus of the Cichlidae. This is possibly the immature form of L. subtriquetra.
ii. L. recurvata, Diesing, found in the frontal sinuses and the trachea of Felis onca.
iii. L. subtriquetra, Diesing, found in the throat of Caiman latirostris and C. sclerops, perhaps the mature form of L. pusilla.
iv. L. taenioides, Lamarck, found in the frontal sinuses and nasal chambers of the dog and ounce, and in the nasal cavities of the wolf, fox, goat, horse, mule, sheep, and man, and in the trachea of the ounce. The immature form has been found in or on the liver of the cat, guinea-pig, and horse; in the lungs of the ox, cat, guinea-pig, porcupine, hare, and rabbit; in the liver and connective tissue of the small intestine of man; and in the mesenteric glands of the ox, camel, goat, sheep, antelope, fallow-deer, and mouse.
v. Porocephalus annulatus, Baird, found in the lungs of the Egyptian cobra, Naja haje; the immature form is thought to live encapsuled in a species of Porphyrio[391] and in the Numidian Crane.
vi. P. aonycis, Macalister, from the lungs of an Indian otter taken in the Indus.
vii. P. armillatus, Wyman, found in the adult state in the lungs of certain African pythons, and in the lion; in the larval form it occurs encysted in the abdomen of the Aard-wolf, the mandril, and man—usually in negroes. Its migrations in the body of its second host sometimes cause fatal results.
viii. P. bifurcatus, Diesing, found in the body-cavity of certain snakes, and in the lungs of boa-constrictors and the legless lizard, Amphisbaena alba. Possibly an immature form.
ix. P. clavatus, Lohrmann, found in the lungs of the Monitor lizard.
x. P. crocidura, Parona, found in the peritoneum of the “musk-rat” Crocidura in Burmah. Probably a larval form.
xi. P. crotali, Humboldt, found in the lungs, body-cavity, kidneys, spleen, and mesentery of many snakes and lizards, and of the lion and leopard. The immature forms occur in the liver and abdominal cavity of species of opossum, armadillo, mouse, raccoon, bat, and marmoset.
xii. P. geckonis, Dujardin, found in the lungs of a Siamese gecko.
xiii. P. gracilis, Diesing, found free in the body-cavity or encapsuled on the viscera and mesenteries of South American fishes, snakes, and lizards,
xiv. P. heterodontis; Leuckart, found encapsuled in the abdominal muscles and mesentery of a species of Heterodon.
xv. P. indicus,[392] v. Linst., found in the trachea and lungs of Gavialis gangeticus.
xvi. P. lari, Mégnin, found in the air-sacs of the Burgomaster or Glaucous gull, Larus glaucus of the Polar seas.
497xvii. P. megacephalus, Baird, found embedded in the flesh of the head of an Indian crocodile, C. palustris, the “Mugger.” Probably a larval form.
xviii. P. megastomus, Diesing, found in the lungs of a fresh-water tortoise, Hydraspis geoffroyana.
xix. P. moniliformis, Diesing, found in the lungs of pythons.
xx. P. najae sputatricis, Leuckart, found encapsuled in the abdominal muscles and peritoneum of the cobra, Naja tripudians. Probably a larval form.
xxi. P. oxycephalus, Diesing, found in the lungs of crocodiles and alligators.
xxii. P. platycephalus, Lohrmann, habitat unknown.
xxiii. P. subuliferus, Leuckart, in the lungs of the cobra Naja haje.
xxiv. P. teretiusculus, Baird, found in the lungs and mouth of certain Australian snakes.
xxv. P. tortus, Shipley, found in the body-cavity of a snake, Dipsadomorphus irregularis, taken in New Britain.
xxvi. Reighardia, sp., Ward, found in the air-sacs of Bonaparte’s gull and the common North American tern.
PYCNOGONIDA
CHAPTER XXI
PYCNOGONIDA[393]
Remote, so far as we at present see, from all other Arthropods, while yet manifesting the most patent features of the Arthropod type, the Pycnogons constitute a little group, easily recognised and characterised, abundant and omnipresent in the sea. The student of the foreshore finds few species and seldom many individuals, but the dredger in deep waters meets at times with prodigious numbers, lending a character to the fauna over great areas.

Fig. 262.—Pycnogonum littorale, Ström, × 2.
The commonest of our native species, or that at least which we find the oftenest, is Pycnogonum littorale (Phalangium littorale, Ström, 1762). We find it under stones near low water, or often clinging louse-like to a large Anemone. The squat segmented trunk carries, on four pairs of strong lateral processes, as many legs, long, robust, eight-jointed, furnished each with a sharp terminal claw. In front the trunk bears a long, stout, 502tubular proboscis, at the apex of which is the mouth, suctorial, devoid of jaws; the body terminates in a narrow, limbless, unsegmented process, the so-called “abdomen,” at the end of which is the anal orifice. The body-ring to which is attached the first pair of legs, bears a tubercle carrying four eye-spots; and below, it carries, in the male sex, a pair of small limbs, whose function is to grasp and hold the eggs, of which the male animal assumes the burden, carrying them beneath his body in a flattened coherent mass. In either sex a pair of sexual apertures open on the second joints of the last pair of legs. The integument of body and limbs is very strongly chitinised, brown in colour, and raised into strong bosses or tubercles along the middle line of the back, over the lateral processes, and from joint to joint of the limbs. The whole animal has a singular likeness to the Whale-louse, Cyamus mysticeti (well described by Fr. Martins in 1675), that clings to the skin of the Greenland Whale as does Pycnogonum to the Anemone, a resemblance close enough to mislead some of the older naturalists, and so close that Linnaeus, though in no way misled thereby, named it Phalangium balaenarum. The substance of the above account, and the perplexity attending the classification of the animal, are all included in Linnaeus’s short description:[394] “Simillimus Onisco Ceti, sed pedes omnes pluribus articulis, omnes perfecti, nec plures quam octo. Dorsum rubrum, pluribus segmentis; singulis tribus mucronibus. Cauda cylindrica, brevissima, truncata. Rostrum membranaceum, subsubulatum, longitudine pedum. Genus dubium, facie Onisci ceti; rostro a reliquis diversum. Cum solo rostro absque maxillis sit forte aptius Acaris aut proprio generi subjiciendum.... Habitat in mari norvegico sub lapidibus.”[395]

Fig. 263.—Dorsal view of Nymphon brevirostre, Hodge, × 6. Britain.
The common Pycnogonum is, by reason of the suppression of certain limbs, rather an outlying member than a typical representative of the Order, whose common characters are more strikingly and more perfectly shown in species, for instance, of Nymphon. Of this multiform genus we have many British species, some of the smaller being common below tide-marks, creeping among weeds or clinging like Caprellae with skeleton limbs to the branches of Zoophytes, where their slender forms are not easily seen. In contrast to the stouter body and limbs of Pycnogonum, the whole fabric of Nymphon tends to elongation; the body is drawn out so that the successive lateral processes stand far apart, and a slender neck intervenes between the oculiferous tubercle and the proboscis; the legs are produced to an amazing length and an extreme degree of attenuation: “mirum tam parvum corpus regere tam magnos pedes,” says Linnaeus. Above the base of the proboscis are a pair of three-jointed appendages, the two terminal joints of which compose a forcipate claw; below and behind these come a pair of delicate, palp-like 504limbs of five joints; and lastly, on the ventral side, some little way behind these, we find the ovigerous legs that we have already seen in the male Pycnogonum, but which are present in both sexes in the case of Nymphon. At the base of the claw which terminates each of the eight long ambulatory legs stands a pair of smaller accessory or “auxiliary” claws. The generative orifices are on the second joint of the legs as in Pycnogonum, but as a rule they are present on all the eight legs in the female sex, and on the two hindmost pairs in the male. One of the Antarctic Nymphonidae (Pentanymphon) and one other Antarctic genus less closely related (Decolopoda) have an extra pair of legs. No other Pycnogon, save these, exhibits a greater number of appendages than Nymphon nor a less number than Pycnogonum, nor are any other conspicuous organs to be discovered in other genera that are not represented in these two: within so narrow limits lie the varying characters of the group.

Fig. 264.—Nymphon brevirostre, Hodge. Head, from below, showing chelophores, palps, and ovigerous leg.
In framing a terminology for the parts and members of the body, we encounter an initial difficulty due to the ease with which terms seem applicable, that are used of more or less analogous parts in the Insect or the Crustacean, without warrant of homology. Thus the first two pairs of appendages in Nymphon have been commonly called, since Latreille’s time, the mandibles and the palps (Linnaeus had called them the palps and the antennae), though the comparison that Latreille intended to denote is long abandoned; or, by those who leaned, with Kröyer and Milne-Edwards, to the Crustacean analogy, mandibles and maxillae. Dohrn eludes the difficulty by denominating the appendages by simple numbers, I., II., III., ... VII., and this method has its own advantages; but it is better to frame, as Sars has done, a new nomenclature. With him we shall speak of the Pycnogon’s body as constituted of a trunk, whose first (composite) segment is the cephalic segment or head, better perhaps the cephalothorax, and which terminates in a caudal segment or abdomen; the “head” bears the proboscis, the first appendages 505or “chelophores,” the second or “palps,” the third, the false or “ovigerous” legs, and the first of the four pairs of “ambulatory” legs. The chelophores bear their chela, or “hand,” on a stalk or scape; the ambulatory legs are constituted of three coxal joints, a femur, two tibial joints, a tarsus, and a propodus, with its claws, and with or without auxiliary claws.
The Body.—The trunk with its lateral processes may be still more compact than in Pycnogonum, still more attenuated than in Nymphon.
In a few forms (e.g. Pallene, Ammothea, Tanystylum, Colossendeis) the last two, or even more, segments of the trunk are more or less coalescent. In Rhynchothorax the cephalic segment is produced into a sharp-pointed rostrum that juts forward over the base of the proboscis. The whole body and limbs may be smooth, tuberculated, furnished with scattered hairs, or sometimes densely hispid.

Fig. 265.—A, Colossendeis proboscidea, Sabine, Britain; B, Ammothea echinata, Hodge, Britain; C, Phoxichilus spinosus, Mont., Arctic Ocean. (The legs omitted.)
The proboscis varies much in shape and size. It may be much longer or much shorter than the body, cylindrical or tumid, blunt or pointed, straight or (e.g. Decolopoda) decurved; usually firmly affixed to the head and pointing straight forwards; sometimes (Eurycide, Ascorhynchus) articulated on a mobile stalk and borne deflexed beneath the body.
Chelophores.—The first pair of appendages or chelophores are wanting in the adult Pycnogonum, Phoxichilus, Rhynchothorax, and Colossendeis.[396]
506In Ammothea and its allies they are extremely rudimentary in the adult, being reduced to tiny knobs in Tanystylum and Trygaeus, and present as small two-jointed appendages in Ammothea; in this last, if not in the others also, they are present in complete chelate form in the later larval stages.

Fig. 266.—A, B, Chelophores of Ascorhynchus abyssi, G.O.S. A, Young; B, adult. (After Sars.) C, Anterior portion of Ammothea hispida, Hodge, Jersey: late larval stage (= Achelia longipes, Hodge), showing complete chelae. D, Chela of Eurycide hispida, Kr.
In Eurycide, Ascorhynchus, and Barana they are usually less atrophied, but yet comparatively small and with imperfect chelae, while in some Ascorhynchi (A. minutus, Hoek) they are reduced to stumps.

Fig. 267.—Chelae of species of Nymphonidae: A, Nymphon brevirostre, Hodge; B, Boreonymphon robustum, Bell; C, Chaetonymphon macronyx, G.O.S.; D, Nymphon elegans, Hansen.

Fig. 268.—Proboscis and chelophores of Cordylochele longicollis, G.O.S. (After Sars.)
In Pallenopsis the scape of the chelophore consists of two joints, as also in Decolopoda and some Ascorhynchus: in Nymphon, Phoxichilidium, Pallene, and Cordylochele of one only; in all 507these the terminal portion or “hand” forms a forcipate “chela,” of which the ultimate joint forms the “movable finger.” In some species of Nymphon the chela is greatly produced and attenuated, and armed with formidable serrate teeth on its opposing edges; in others it is shortened, with blunter teeth; in Boreonymphon robustum the claws are greatly curved, with a wide gap between. In this last, and in Phoxichilidium, the opposing edges are smooth and toothless. In Cordylochele the hand is almost globular, the movable finger being shortened down, and half enclosed by the other.

Fig. 269.—Eurycide hispida, Kr., showing stalked proboscis and zigzag palps.
Palpi.—The second pair of appendages, or palps, are absent, or all but absent, in the adult Pycnogonum, Phoxichilus, Phoxichilidium, Pallene, and their allies. In certain of these cases, e.g. Phoxichilidium, a knob remains to mark their place; in others, e.g. Pallenopsis, a single joint remains; in a few Pallenidae a sexual difference is manifested, reduction of the appendage being carried further in the female than in the male. The composition of the palps varies in the genera that possess them. In Nymphon there are five joints, and their relative lengths (especially of the terminal ones) are much used by Sars in defining the many species of the genus. The recently described Paranymphon, Caullery, has palps of six or seven joints. In the Ammotheidae the number of joints ranges from five or six in Tanystylum to nine (as a rule) in Ammothea and Oorhynchus, or ten, according to Dohrn, in certain species of Ammothea. Colossendeis and the Eurycididae have a ten-jointed palp, which in this last family is very long and bent in zigzag fashion, as it is, by the way, also in Ammothea. The terminal joints of the palp are in all cases more or less setose, and their function is conjecturally tactile.
Ovigerous Legs.—Custom sanctions for these organs an inappropriate name, inasmuch as it is only in the males that they perform the function which the name connotes.[397] They 508probably also take some part, as Hodgson suggests, in the act of feeding.

Fig. 270.—Ovigerous legs of A, Phoxichilus spinosus, Mont.; B, Phoxichilidium femoratum, Rathke; C, Anoplodactylus petiolatus, Kr.; D, Colossendeis proboscideus, Sab.

Fig. 271.—Terminal joints of ovigerous leg of Rhynchothorax mediterraneus, Costa.

Fig. 272.—Nymphon brevirostre, Hodge. Terminal joints of ovigerous leg, with magnified “tooth.”
In Pycnogonum, Phoxichilus, Phoxichilidium, and their immediate allies they are absent in the female; in all the rest they are alike present in both sexes, though often somewhat smaller in the female than in the male. They are always turned towards the lower side of the body, and in many cases even their point of origin is wholly ventral. The number of joints varies: in Phoxichilidium five, Anoplodactylus six, Phoxichilus seven; in Paranymphon eight; in Pycnogonum nine, with, in addition, a terminal claw; in the Ammotheidae from seven (Trygaeus) to ten, without a claw; in Pallenidae ten, with or without a claw; in Rhynchothorax, Colossendeis, Eurycide, Ascorhynchus, Nymphon, ten and a claw. The appendage, especially when long, is apt to be wound towards its extremity into a spiral, and its last four joints usually possess a peculiar armature. In Rhynchothorax this takes the form of a stout toothed tubercle on each joint; in Colossendeis of several rows of small imbricated denticles; in Nymphon and Pallene of a single row of curious serrate and pointed spines, each set in a little membranous socket.

Fig. 273.—Nymphon strömii, Kr. Male carrying egg-masses on his ovigerous legs.

Fig. 274.—Terminal joints (tarsus and propodus) of legs. 1, Chaetonymphon hirtum, Fabr.; 2, N. strömii, Kr.; 3, Nymphon brevirostre, Hodge; 4, Ammothea echinata, Hodge; 5, Ascorhynchus abyssi, G.O.S. (All after Sars.)
Legs.—The four pairs of ambulatory legs are composed, in all cases without exception, of eight joints if we exclude, or nine if we include, the terminal claw. They vary from a length about equal to that of the body (Pycnogonum, Rhynchothorax, Ammothea) to six or seven times as much, perhaps more, in Nymphon and Colossendeis, the fourth, fifth, and sixth joints being those that suffer the greatest elongation. The seventh joint, or tarsus, is usually short, but in some Nymphonidae is much elongated; the eighth, or propodus, is usually somewhat curved, and usually possesses a special armature of simple or serrate spines. The 510auxiliary claws, sometimes large, sometimes small, lie at the base of the terminal claw in Ammotheidae, Phoxichilidae, in Phoxichilidium, in most Pallenidae, in nearly all Nymphonidae. Their presence or absence is often used as a generic character, helping to separate, e.g., Pallene from Pseudopallene and Pallenopsis, and Phoxichilidium from Anoplodactylus; nevertheless they may often be detected in a rudimentary state when apparently absent. The legs are smooth or hirsute as the body may happen to be.

Fig. 275.—Legs of A, Pallene brevirostris, Johnston; B, Anoplodactylus petiolatus, Kr.; C, Phoxichilus spinosus, Mont.; D, Colossendeis proboscidea, Sabine; E, Ammothea echinata, Hodge, ♂.

Fig. 276.—Boreonymphon robustum, Bell. Male with young, slightly enlarged. Faeroe Channel.
Glands.—In some or all of the appendages of the Pycnogonida may be found special glands with varying and sometimes obscure functions. The glands of the chelophores (Fig. 280, p. 522) are present in the larval stages only. They consist of a number of flask-shaped cells[398] lying within the basal joint of the appendage, and generally opening at the extremity of a long, conspicuous, often mobile, spine (e.g. Ammothea (Dohrn), Pallene, Tanystylum (Morgan), Nymphon brevicollum and N. gracile (Hoek)). They secrete a sticky thread, by means of which the larvae attach 512themselves to one another and to the ovigerous legs of the male parent. In Nymphon hamatum, Hoek, the several filaments secreted by the separate sacculi of the gland issue separately. In Pycnogonum the spine on which the gland opens is itself prolonged into a long fine filament, and here, according to Hoek, the gland is in all probability functionless and rudimentary. Hoek has failed to find the gland in Ascorhynchus, and also in certain Nymphonidae (e.g. Boreonymphon robustum, Bell), in which the young are more than usually advanced at the time of hatching. The gland has also been described by Lendenfeld and others in Phoxichilidium, whose larvae do not cling together but live a parasitic life; in this genus the long spine or tubercle is absent on which the orifice is usually situated, and, according to Lendenfeld, the secretion issues from many small orifices set along the opposing edges of the chela. Of the two species described by Dohrn as Barana castelli and B. arenicola, the former has the spine of inordinate length, more than twice as long as the whole body, chelophore and all; while in the latter (which species rather resembles Ascorhynchus) the spine is altogether absent.
In the palps and ovigerous legs of the adult are found glandular bodies of a hollow vesicular form with a simple lining of cells, the vesicle being divided within by a septum with a central orifice, the outer and smaller half opening to the exterior. These glands are probably of general occurrence, but they have been but little investigated. They lie usually in the fourth and fifth joints of the palp, and the third and fourth joints of the ovigerous leg. Hoek describes them in Discoarachne (Tanystylum) as lying within the elongated third joint of the palp, and opening by a sieve-plate at the end of the second joint. In Ammothea (Dohrn) and Ascorhynchus (Hoek) they open on a small tubercle situated on the fifth joint of the palp. In Nymphon, Hoek describes them as opening by a small pore on the fourth joint of the ovigerous leg. Dohrn failed to find them in Pycnogonum, but in Phoxichilus, Phoxichilidium and Pallene he discovered the glands appertaining to the palps, though the palps themselves have disappeared in those genera; he has found the glands also in Ammothea, in larvae that have not yet attained their full complement of legs.
The males in nearly all cases are known to possess glands in 513the fourth joints or thighs of all the ambulatory legs, and these glands without doubt act as cement-glands, emitting, like the chelophoral glands of the larvae, a sticky thread or threads by which the eggs and young are anchored to the ovigerous legs. In some species of Nymphon and of Colossendeis Hoek could not find these, and he conjectures them to be conspicuous only in the breeding season. While in most cases these glands open by a single orifice or by a few pores grouped closely together, in Barana, according to Dohrn, and especially in B. arenicola, the pores are distributed over a wide area of the femoral joint.[399] In Discoarachne (Loman) and Trygaeus they open into a wide chitinised sac with tubular orifice. While the function of these last glands and of the larval glands seems plain enough, that of those which occur in the palps and ovigerous legs of both sexes remains doubtful.
In their morphological nature the two groups of glands are likewise in contrast, the former being unicellular glands, such as occur in various parts of the integument of the body and limbs of many Crustacea; while the latter are segmentally arranged and doubtless mesoblastic in origin, like the many other segmental excretory organs (or coelomoducts) of various Arthropods.
By adding colouring matters (acid-fuchsin, etc.) to the water in which the animals were living, Kowalevsky demonstrated the presence of what he believed to be excretory organs in Phoxichilus, Ammothea, and Pallene. These are small groups of cells, lying symmetrically near the posterior borders of the first three body-segments, and also near the bases of the first joints of the legs, dorsal to the alimentary canal.[400]

Fig. 277.—Longitudinal section through one “antimere” of the proboscis in Phoxichilus charybdaeus. G, g′, Principal and secondary ganglia; h, sieve-hairs; L, lip; mt, oral tooth; N, N′, inner and outer nerve-cords; t, proboscis-teeth. (After Dohrn.)
Alimentary System.—The proboscis is a very complicated organ, and has been elaborately described by Dohrn.[401] It is a prolongation of the oral cavity, containing a highly developed stomodaeum, but showing no sign of being built up of limbs or 514gnathites. The mouth, situated at its apex, is a three-sided orifice, formed by a dorsal[402] and two lateral lobes; and hence the proboscis has been assumed by some, on no competent evidence, to be constituted of a degenerate pair of appendages and a labrum or upper lip. Each of the three lobes which bounds the mouth shows the following structures: firstly, a lappet of external chitinised integument, overlapping, as the finger-nail overlaps the finger, a cushion-like lip, ridged after the fashion of a fine-cut file in some species, hairy in others, on the inner surface where the three lips meet to close the orifice of the mouth. Below this again is a prominent tooth (Fig. 277, mt), supported, as are the lips, by a system of chitinous rods, which are but little developed in the genus here figured, though conspicuous and complicated in others. Transverse ridges run across the angles where adjacent lips meet, and the whole mechanism constitutes an efficient valve, preventing the escape of swallowed food. The greater portion of the proboscis is occupied by a masticating or triturating apparatus, the oesophageal cavity expanding somewhat and having its walls densely covered, in three bands corresponding to the antimeres, with innumerable minute spines (h) or needles, sometimes supplemented by large teeth (t) that point forwards somewhat obliquely to the axis of the proboscis.[403]
In the curious East Indian genus Pipetta (Loman) the sucking and sifting mechanism is low down in the proboscis, and the organ is prolonged into a very fine tube, the lips growing together till they leave an aperture of only ·007 mm. for the absorption of liquids.
515In some cases, where the proboscis itself is short, as in Pallene, this mechanism is carried backwards into the fore-part of the body; and, in the latter genus, the narrow oesophagus which succeeds the masticatory apparatus is likewise provided with extrinsic muscles.

Fig. 278.—Transverse sections through the proboscis of Ph. charybdaeus. A, Anterior, through the principal ganglionic mass (G); B, posterior, at the level of the sieve-hairs (h). Coec, Intestinal caeca; Dil. M, dilator muscles; N, inner nerve-ganglion, with circular commissure; N′, outer nerve; or, chitinous lining of oral cavity; R M, Ret.M, retractor muscles. (After Dohrn.)

Fig. 279.—Transverse section through the basal joint of the third leg in Phoxichilus charybdaeus, ♀. Cut, Cuticle; Hyp, hypodermis; Int, intestinal caecum; N, nerve-cord; Ov, ovary; Sept, septum. (After Dohrn.)
The oesophagus is followed by a long gastric cavity, which sends forth caecal diverticula into the chelophores (when these are present), and four immensely long ones into the ambulatory legs. The caeca are attached to the walls of the limb cavities, especially at their extremities in the tarsi, by suspensory threads of connective tissue, and the whole gut, central and diverticular, is further supported by a horizontal septal membrane, running through body and legs, which separates the dorsal blood-vessel and sinus from the gut, the nervous system and the ventral sinus, giving support also to the reproductive glands. A short and simple rectum follows the gastric cavity.
In Phoxichilus, which lacks the three anterior appendages in the female and the two anterior in the male, two pairs of caeca run from the gut into the cavity of the proboscis (Fig. 278, B, coec.).[404]
516Circulatory System.—The heart has been especially studied by Dohrn in Phoxichilus. It consists of a median vessel running from the level of the eyes to the abdomen, furnished with two pairs of lateral valvular openings, and sometimes, though not always, with an unpaired one at the posterior end. The walls are muscular, but with this peculiarity that the muscular walls do not extend around the heart dorsally, in which region its lumen is only covered by the hypodermis and cuticle of the back. The blood-spaces of the body are separated into dorsal and ventral halves by the septal membrane already referred to, which is perforated in the region of the lateral processes by slits placing the two cavities in communication; this septal membrane runs through the limbs to their tips, and far into the proboscis, where it is attached to the edge of the superior antimere. The blood is a colourless plasma with several kinds of corpuscles, of which the most remarkable are amoeboid, actively mobile, often coalescing into plasmodia. The course of the circulation is on the whole outwards in the inferior or ventral sinus, inwards towards the heart in the superior, save in the proboscis, where the systole of the heart drives the blood forwards in the dorsal channel. The beat is rapid, two or three times in a second, according to Loman, in Phoxichilidium. Especially in the species with small body and exaggerated legs, the movement of the circulatory fluid is actuated more by the movements of the limbs and the contractions of the intestinal caeca than by the direct impulse of the heart.
Nervous System.—The nerve-chain consists of a fused pair of supra-oesophageal ganglia, which innervate (at least in the adult) the chelophores, and of ventral ganglia, whence proceed the nerves to the other limbs. The ganglia of the second and third appendages are fused with one another, sometimes also with the ganglia of the first ambulatory legs; the ganglia of the three posterior pairs of legs are always independent (though the development of their longitudinal commissures varies with the body-form), and they are succeeded by one or two pairs of ganglia, much reduced in size, situated in the abdomen, of which the posterior one innervates the muscles of the abdomen and of the anal orifice. Each lateral nerve divides into two main branches, which supply the parts above and below the septal membrane. The nerve-supply of the proboscis is very complicated. 517Its upper antimere is supplied from the pre-oral, its two lateral antimeres from the first post-oral, ganglion, and each of these three nerves divides into two branches, of which the inner bears six to eight or more small ganglia, which annular commissures passing round the pharynx connect one to another. Of these ganglia and commissures the anterior are the largest, and with these the outer lateral nerve-branches of the proboscis merge. The immediate origin of the nerves to the chelophores is from the median nerve that springs from the under side of the supra-oesophageal ganglion to run forward into the proboscis, but it is noteworthy that the chelophores receive twigs also from the lateral nerves of the proboscis which arise from the post-oral ganglia.
Eyes.—Eyes are the only organs of special sense known in the Pycnogons. The deep-water Pycnogons, in general those inhabiting depths below four or five hundred fathoms, have in most cases imperfect organs, destitute of lens and of pigment, so imperfect in many cases as to be described as wanting. It is rare for the eyes to be lacking in shallow-water species, as they are, for instance, in Ascorhynchus minutus, Hoek, dredged by the Challenger in 38 fathoms, but, on the other hand, it is no small minority of deep-water species that possess them of normal character and size, even to depths of about 2000 fathoms.
In all cases where eyes are present, they are simple or “monomeniscous” eyes, four in number, and are situated in two pairs on an “oculiferous tubercle,” sometimes blunt and low, sometimes high and pointed, placed on the so-called cephalothorax, or first, compound, segment of the body. The anterior pair are frequently a little larger, sometimes, as in Phoxichilidium mollissimum, Hoek, very much larger, than the posterior. The minute structure of the eye has been investigated by Dohrn, Grenacher, Hoek, and Morgan. The following account is drawn in the first instance from Morgan’s descriptions.[405]
The eye of a Pycnogon (Phoxichilidium) is composed of three layers, an outer layer of specialised ectoderm cells (hypodermis) that secrete the cuticular lens, a middle layer of visual or retinal elements, and an inner layer of pigment-cells. The elements of the middle layer consist of much elongated cells, whose branching outer ends are connected with nerve-fibrils and 518interwoven in a protoplasmic syncytium, whose middle parts are occupied by the nuclei and whose inwardly directed ends form the retinal rods or bacilli. The pigment-cells of the inner layer are of various forms, those towards the middle of the eye being small and flattened, those at the sides being, for the most part, long and attenuated, so seeming, as Morgan remarks, to approximate in character to the retinal elements. The pigment-layer is easily dispersed and reveals beneath it a median vertical raphe, caused by the convergence of the cells of the middle layer from either side, and along the line of this raphe the optic nerve joins the eye, though its subsequent course to its connection with the retinal elements is obscure. It is at least clear that the retina is an “inverted” retina, with the nerve-connected bases of its cells lying outwards and their bacillar extremities directed inwards.
In a longitudinal vertical section of the eye of a larva (Tanystylum), at a stage when three pairs of walking legs are present, Morgan shows us the pigment-layer apparently continuous with the hypodermis just below the eye, and in close connection with the middle layer at the upper part of the eye. From this we are permitted to infer a development by invagination, in which the long invaginated sac is bent and pushed upwards till it comes into secondary contact with the hypoderm, so giving us the three layers of the developed eye. This manner of formation is precisely akin to that described by Parker, Patten, Locy, and others for the median eyes of Scorpions and of Spiders, and the organ is structurally comparable to the Nauplius- or median eye of Crustacea. But neither in these cases nor in that of the Pycnogon is the whole process clear, in consequence chiefly of the obscurity that attends the course of the optic nerve in both embryo and adult. For various discussions and accounts, frequently contradictory, of these phenomena, the reader is referred to the authors quoted, or to Korschelt and Heider’s judicious summary.[406]
There seems to be a small structure, of some sort or other, between the ocelli on either side. Dohrn thought it might be auditory, Loman that it might be secretory, but its use is unknown.
Integument.—The chitinised integument is perforated by 519many little cavities, some of them conical and tapering to a minute external pore, the others more regularly tubular. Sometimes, but according to Hoek rarely, the tubular pore-canals communicate with, or arise from, the conical cavities. The pore-canals transmit a nerve for the supply of sensory hairs, often forked, which arise from the orifice of the canal in little groups of two or more, sometimes in rosettes of eight or nine. These setae are small or rudimentary in Ascorhynchus and totally wanting in Colossendeis; they appear to be extremely large and stellate in Paranymphon. The conical cavities contain proliferated epithelial cells, blood corpuscles, and cells of more doubtful nature that are perhaps glandular. According to Dohrn, glands exist in connection with both kinds of integumentary perforations, and he suspects that they secrete a poisonous fluid in response to stimuli affecting the sensory hairs; Hoek, on the other hand, is inclined to ascribe a respiratory function to the cavities; but indeed, as yet, we must confess that their use is undetermined.
Reproductive Organs.—In each sex the generative organs consist of a pair of ovaries or testes lying above the gut on either side of the heart; in the adult they are fused together posteriorly at the base of the abdomen, and send long diverticula into the ambulatory legs. In the female Phoxichilidium, at least, as Loman has lately shown, the fusion is complete, and the ovary forms a thin broad plate, spreading through the body and giving off its lateral diverticula. The diverticula of the testes reach to the third joint of the legs, those of the ovaries to the fourth, or sometimes farther. The ova ripen within the lateral diverticula, chiefly, and sometimes (Pallene) exclusively, in the femora or fourth joints of the legs,[407] which, in many forms, are greatly swollen to accommodate them; the spermatozoa, on the other hand, are said to develop both within the legs and within the thoracic portions of the testis. The genital diverticula may end blindly within the leg, or communicate through a duct with the exterior by a valvular aperture placed on the second coxal joint. Such apertures occur, as a rule, on all the legs in the females, in Rhynchothorax and Pycnogonum on the last only. In the males an aperture is present on all the legs in Decolopoda and Phoxichilidium; on the last three in Nymphon and Phoxichilus; 520in most genera on the last two; in Pycnogonum and Rhynchothorax on the last only.
Very commonly the female individuals are somewhat larger than the males, and in some species (Ammothea, Trygaeus) the latter are distinguished by a greater development of spines or tubercles on the body and basal joints of the legs (Dohrn).
The act of fecundation has been observed by Cole[408] in Anoplodactylus. The animal reproduces towards the end of August. Consorting on their Eudendrium (Hydroid) colony, the male climbs upon the female and crawls over her head to lie beneath her, head to tail; and then, fertilisation taking place the while, the hooked ovigerous legs of the male fasten into the extruding egg-masses and tear them away. The whole process is over in five minutes. The fresh egg-masses are more or less irregular in shape, and white in colour like little tufts of cotton.
Each ball of eggs that the male carries represents the entire brood of one female, and in Phoxichilidium Loman has seen a male carrying as many as fourteen balls. Fertilisation is external, taking place while the eggs are being laid. The spermatozoa have small rounded heads and long tails, and are thus unlike the spermatozoa of most Crustacea.
Development.—Until the hatching of the embryo, the eggs of the Pycnogons are carried about, agglutinated by cement-substance into coherent packets, on the ovigerous legs of the males. They are larger or smaller according to the amount of yolk-substance present, very small in Phoxichilidium and Tanystylum (Morgan), where they measure only ·05 mm. in diameter; larger in Pallene (·25 mm.); larger still (·5–·7 mm.) in Nymphon. In Pallene each egg-mass commonly contains only two eggs; in the other genera they are much more numerous, rising to a hundred or more in Ammothea (Dohrn). The egg-masses may be one or more on each ovigerous leg, sometimes (Phoxichilidium angulatum, Dohrn) a single egg-mass is held by both legs; they are extremely numerous in Phoxichilus, and in Pycnogonum they coalesce to form a broad pad beneath the body. The fact that it is the male and not the female that carries the eggs was only announced in 1877 by Cavanna;[409] 521before, and by some even after his time, the two sexes were constantly confused.[410]

Fig. 280.—Young larva (nat. size ·1 mm.) of Ammothea fibulifera, Dohrn. C.G, Brain; gl, gld, gland and duct of chelophore; pr, proboscis; I, II, III, IV, appendages. (After Dohrn.)
Segmentation is complete, symmetrical in the forms with smaller eggs, unequal in those burdened with a preponderance of yolk (Morgan). In Pallene, as in the Spider’s egg, what is described as at first a total segmentation passes into a superficial or centrolecithal one by the migration outwards of the nuclei and the breaking down of the inner ends of the wedge-shaped segmentation-cells. The blastoderm so formed becomes concentrated at the germinal pole of the egg. A thickened portion of the blastoderm (which Morgan compares to the “cumulus primitivus” of the Spider’s egg) forms an apparently blastoporal invagination (though Morgan calls it the stomodaeum), and from its sides are budded off the mesodermal bands. Meisenheimer has recently given a minute account of the early development of Ammothea, a form with small yolkless eggs. Here certain cells of the uniform and almost solid blastosphere grow inwards till their nuclei arrange themselves in an inner layer of what (so far as they are concerned) is a typical gastrula, but without any central cavity. The inner layer subsequently, but slowly, differentiates into the mid-gut, and into dorsal and lateral offshoots, the sources of the heart and of the muscles and connective tissues respectively. The further development of the egg takes place, as is usual in Arthropods, by the appearance, in a longitudinal strip or germ-band which enwraps the yolk, of paired thickenings which represent the cerebral and post-oral ganglia, and of others from which arise the limbs. Of these latter, the chelophores are the first to appear, on either side of the mouth; in Pallene the fourth pair appears next in order, followed by the fifth and sixth, and by the third and seventh just before the hatching out of the embryo; the second is lacking in this particular genus. Thus in Pallene (Dohrn, Morgan), and in some others, e.g. Nymphon brevicollum (Hoek), the free larva is from the first provided with its full complement of limbs. Certain other species of Nymphon hatch out in possession of four or five pairs of limbs, but in the great 522majority of cases studied the larval Pycnogon is at first provided with three pairs only, the three anterior pairs of the typical adult.[411] Numerical coincidence, and that alone, has often led this “Protonymphon” larva to be compared with the Crustacean Nauplius. In the annexed figure of a young larval Ammothea (Achelia), we see the unsegmented body, the already chelate chelophores (furnished with the provisional cement-glands already described), the other two pairs of appendages each with a curious spine at its base, the gut beginning to send out diverticula (of which the first pair approach the chelophores) but still destitute of the anus (which is only to be formed after the development of the abdomen), the proboscis, and one pair of eyes situated close over the pre-oral ganglia. The subsequent changes are in this genus extremely protracted, and terminate with the loss of the chelae, a process which occurs so late in life that the chelate individuals were long looked upon as belonging to a separate genus, the original Ammothea of Hodge, until Hoek proved their identity with the clawless Achelia.
The developmental history of Phoxichilidium and Anoplodactylus is peculiar. The young larvae have the claws of the second and third appendages hypertrophied to form enormous stiff tendril-like organs, with which they affix themselves to the bodies of Hydroid Zoophytes (Coryne, Eudendrium, Tubularia, Hydractinia, 523etc.), feeding as the adults do: afterwards losing these elongated tendrils in a moult, they pass into the gastral cavity of the Hydroid; in our native species the larva issues from the Hydroid and begins its independent life at a stage when three pairs of ambulatory legs are present and the fourth is in bud.[412] The Phoxichilidium larvae were first noticed by Gegenbaur in Eudendrium,[413] again by Allman in Coryne eximia.[414] George Hodge made detailed and important observations,[415] and showed, in opposition to Gegenbaur, that it was the larva which entered the Hydroid and not the egg that was laid therein.[416]

Fig. 281.—Larva of Phoxichilidium sp., showing tendril-like appendages of the larval palps and ovigerous legs. (After Dohrn.)
Moseley has the following interesting note in his Challenger Report:[417] “The most interesting parasite observed was a form found in the gastric cavities of the gastrozoids of Pliobothrus symmetricus (West Indies, 450 f.), contained in small capsules. These capsules were badly preserved, but there seemed little 524doubt that they contained the remains of larvae of a Pycnogonid, so that the deep-sea Pycnogonids, which are so abundant, very possibly pass through their early stages in deep-sea Stylasteridae.... The gastrozoids containing the larvae were partly aborted.”
A Pycnogon larva, doubtfully ascribed to Nymphon, has been found living in abundance ectoparasitically on Tethys in the Bay of Naples.[418]
Habits.—Of the intimate habits of the Pycnogons we can say little. Pycnogonum we often find clinging, as has been said, close appressed to some large Anemone (Tealia, Bolocera, etc.), whose living juices it very probably imbibes. The more slender species we find climbing over sea-weeds and Zoophytes, where sometimes similarity of colour as well as delicacy of form helps to conceal them; thus Phoxichilidium femoratum (Orithyia coccinea, Johnston) is red like the Corallines among which we often find it, P. virescens green like the filamentous Ulvae, the Nymphons yellowish like the Hydrallmania and other Zoophytes which they affect. On the New England coast, according to Cole, the dark purple Anoplodactylus lentus, Wilson (Phoxichilidium maxillare, Stimpson), is especially abundant on colonies of Eudendrium, whose colour matches its own, the yellowish Tanystylum orbiculare frequents a certain yellowish Hydroid, and of these two species neither is ever found on the Hydroid affected by the other; while, on the other hand, Pallene brevirostris, whose whitish, almost transparent body is difficult to see, is more generally distributed.[419] The deep-sea Pycnogons (Colossendeis, Nymphon) are generally (if not universally) of a deep orange-scarlet colour, a common dress of many deep-sea Crustacea.
The movements of the Pycnogons are singularly slow and deliberate; they are manifestly not adapted to capture or to kill a living prey. Linnaeus accepted from J. C. König the singular statement that they enter and feed upon bivalve shells, “Mytilorum testes penetrat et exhaurit”; but the statement has never been reaffirmed.[420]
525Loman describes Phoxichilidium as feeding greedily on Tubularia larynx, and especially on the gonophores. It grasps them with its claws, sucks them in bit by bit till the proboscis is filled as far as the sieve, whereupon that part of the proboscis squeezes and kneads the mass, letting only juices and fine particles pass through into the alimentary canal. The lateral caeca and the rectum are separated by sphincter muscles from the stomach; the former are in turn filled with food and again emptied; the contents of the alimentary canal are in constant rolling movement, and the faeces are eliminated by the action of a pair of levatores ani, in round pellets.
The Pycnogons, or some of them, can swim by “treading water,” and Pallene is said by Cole to swim especially well; they more often progress half by swimming, half by kicking on the bottom. They move promptly towards the light, unless they have Hydroids to cling to, and Cole points out that when they crawl with all their legs on the bottom they move forwards towards the light,[421] but backwards when they swim in part or whole. The legs move mostly in a vertical plane, horizontal movements taking place chiefly between the first and second joints. Tanystylum is uncommonly sluggish and inert; it sinks to the bottom, draws its legs over its back and remains quiet, while Pallene, by vigorous kicks, remains suspended.
The long legs of the Pycnogons are easily injured or lost, and easily repaired or regenerated. This observation, often repeated, is as old as Fabricius: “Mutilatur etiam in libertate sua, redintegrandum tamen; vidi enim in quo pedes brevissimi juxta longiores enascentes, velut in asteriis cancris aliisque redintegratis.” In such cases of redintegration of a leg, the reproductive organ, the genital orifice, and the cement-gland are not restored until the next moult.[422]
Systematic Position.—To bring this little group into closer accord with one or other of the greater groups of Arthropods is a problem seemingly simple but really full of difficulty.
The larval Pycnogon, with its three pairs of appendages, resembles the Crustacean Nauplius in no single feature save 526this unimportant numerical coincidence; nor is there any significance in the apparent outward resemblance to isolated forms (e.g. Cyamus) that induced some of the older writers, from Fabricius downwards and including Kröyer and the elder Milne-Edwards, to connect the Pycnogons with the Crustacea. To refer them, or to approximate them to the Arachnids, has been a stronger and a more lasting tendency.[423] Linnaeus (1767) included the two species of which he was cognisant in the genus Phalangium, together with P. opilio. Lamarck, who first formulated the group Arachnida (1802), let it embrace the Pycnogons; and Latreille (1804, 1810), who immediately followed him, defined more clearly the Pycnogonida as a subdivision of the greater group, side by side with the subdivision that corresponds to our modern Arachnida (“Arachnides acères”), and together with a medley of lower Crustacea, Myriapoda, Thysanura, and Parasitic Insects; he was so cautious as to add “j’observerai seulement, que je ne connais pas encore bien la place naturelle des Pycnogonides et des Parasites,” and Cuvier, setting them in a similar position, adds a similar qualification.[424]
Leach (1814), whose great service it was to dissociate the Edriophthalmata and the Myriapoda from the Latreillian medley, left the group Arachnida as we still have it (save for the inclusion of the Dipterous Insect Nycteribia), and divided the group (with the same exception) into four Orders of which the Podosomata, i.e. the Pycnogonida, are one. Savigny (1816), less philosophical in this case than was his wont, assumed the Crustacean type to pass to the Arachnidan by a loss of several anterior pairs of appendages, and appears to set the Pycnogons in an intermediate grade, marking the pathway of the change. He considered the seven pairs of limbs of the Pycnogons to represent thoracic limbs of a Malacostracan, and, like so many of his contemporaries, was much biased by the apparent resemblance of Cyamus to Pycnogonum. The reader may find in Dohrn’s Monograph a guide to many other opinions and judgments, some of them of no small morphological interest and historical value[425]; but it behoves us to pass 527them by, and to inspect, in brief, the case as it stands at present. The obvious features in which a Pycnogon resembles a Spider or other typical Arachnid, are the possession of four pairs of walking legs, and the pre-oral position and chelate form of the first pair of appendages; we may perhaps also add, as a more general feature of resemblance, the imperfect subservience of limbs to the mouth as compared with any of the Crustacea. The resemblance would still be striking, in spite of the presence of an additional pair of legs in a few Pycnogons, were it not for the presence of the third pair of appendages or ovigerous legs of the Pycnogon, whose intercalation spoils the apparent harmony. We are neither at liberty to suppose, with Claus, that these members, so important in the larva, have been interpolated, as it were, anew in the Pycnogon; nor that they have arisen by subdivision of the second pair, as Schimkewitsch is inclined to suppose; nor that they have dropped out of the series in the Arachnid, whose body presents no trace of them in embryo or adult. In a word, their presence precludes us from assuming a direct homology between the apparently similar limbs of the two groups,[426] and at best leaves it only open to us to compare the last legs of the Pycnogon with the first abdominal, or genital, appendages of the Scorpion and the Spider. On the other hand, if we admit the seventh (as we must admit the occasional eighth) pair of appendages of Pycnogons to be unrepresented in the prosoma of the Arachnids, then, in the cephalothorax of the former, with its four pairs of appendages, we may find the homologue of the more or less free and separate part of the cephalothorax in Koenenia, Galeodes, and the Tartaridae. There is a resemblance between the two groups in the presence of intestinal diverticula that run towards or into the limbs, as in Spiders and some Mites, and there are certain histological and embryological resemblances that have been in part referred to above; but these, such as they are, are not adequate guides to morphological classification. We must bear in mind that such resemblances as the Pycnogons 528seem to show are not with the lower Arachnids but with the higher; they are either degenerates from very advanced and specialised Arachnida, or they are lower than the lowest. Confronted with such an issue, we cannot but conclude to let the Pycnogons stand apart, an independent group of Arthropods[427]; and I am inclined to think that they conserve primitive features in the usual presence of generative apertures on several pairs of limbs, and probably also in the non-development of any special respiratory organs. But inasmuch as the weight of evidence goes to show that subservience of limbs to mouth is a primitive Arthropodan character, the fact that the basal elements of the anterior appendages have here (as in Koenenia) no such relation to the mouth must be taken as evidence, not of antiquity, but of specialisation. In like manner the suctorial proboscis cannot be deemed a primitive character, and the much reduced abdomen also is obviously secondary and not primitive.
Classification.—No single genus more than another shows signs of affinity with other groups, and no single organ gives us, within the group, a clear picture of advancing stages of complexity. On the contrary, the differences between one genus and another depend very much on degrees of degeneration of the anterior appendages, and we have no reason to suppose that these stages of degeneration form a single continuous series, but have rather reason to believe that degeneration has set in independently in various ways and at various points in the series. But while we are unable at present to form a natural classification[428] of the Pycnogons, yet at the same time a purely arbitrary or artificial classification, conveniently based on the presence or absence of certain limbs, would run counter to such natural relationships as we can already discern.
529The classification here adopted is a compromise between a natural system, so far as we can detect it, and an artificial one.
Two forms, separated from one another by many differences, show a minimum of degeneration, namely Decolopoda on the one hand, and the Nymphonidae on the other. The former genus has five pairs of legs, and this peculiarity is shared by Pentanymphon. In both groups the three anterior limbs are all present and well formed, save only that the ovigerous legs, which have ten joints in Decolopoda, are reduced to five joints in the Nymphons, and their denticulate spines, of which several rows are present in the former, are reduced to one row in the latter; on the other hand, a greater or a less degeneration of these limbs marks each and all of the other families.
Decolopoda is very probably the most primitive form known, though it has characters which seem to be the reverse of primitive in the dwarfish size of its chelophores and the crowded coalescent segmentation of the trunk. Colossendeis, in spite of its vanished chelophores, is probably closely allied: the shape and segmentation of the body and the several rows of smooth denticles on the ovigerous legs are points in common. The Eurycydidae are closely allied to Colossendeidae; they agree with Decolopoda in the two-jointed scape of the chelophore, and with Ammotheidae in the deflexed mobile proboscis. The true position of Rhynchothorax is very doubtful.
The Nymphonidae and Pallenidae are closely allied, and the Phoxichilidiidae have points of resemblance, especially with the latter. Nymphon compares with Decolopoda in the completeness of its parts, and is more typical in its long well-segmented body, and in its highly-developed chelae; but it already shows reduction in the scape of the chelophore, in the palps, and in the armature of the ovigerous legs.
The Phoxichilidae and Pycnogonidae (Agnathonia, Leach; Achelata, Sars), though differing greatly in aspect, are not improbably allied to one another; and whether this be so or not, the complete absence of chelophores and of palps affords an arbitrary character by which they are conveniently separated from all the rest.
The following table epitomises the chief characters of the several families:—
| Pycnogonida. | Proboscis. | Chelophores. | Palps. | Ovigerous legs. | Teeth on do. | Legs. | Trunk-segments. | Genital Openings. | |
|---|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | ||||||||
| (Cryptochelata, Sars)— | |||||||||
| Decolopodidae | Fixed, decurved | Complete, small, scape 2–jointed | 10 joints | 10 joints ♂, ♀ | Four rows, simple | 5 | Condensed, coalescent | 1, 2, 3, 4, 5, | 1, 2, 3, 4, 5 |
| Colossendeidae | Somewhat mobile, sometimes decurved | 0 | 10 | 10 ♂, ♀ | Many rows, simple | 4 | Coalescent | 1, 2, 3, 4 | 1, 2, 3, 4 |
| Eurycididae | Mobile, stalked, deflexed | Scape 2–jointed, chelae rudimentary | 10 | 10 ♂, ♀ | More than one row, serrate | 4 | Well segmented | 3, 4 | 1, 2, 3, 4 |
| Hannonia | „ | Rudimentary | 0 | 10 ♂, ♀ | Scattered spines | 4 | „ | „ | „ |
| Ammotheidae | Mobile, deflexed | „ | 4–9 | 10 (or less) ♂, ♀ | Few, scattered, serrate or smooth | 4 | Condensed, segmented | „ | „ |
| ? Rhynchothoracidae | Large, fixed, aberrant | 0 | 8 (5) | 10 ♂, ♀ | Toothed tubercles | 4 | „ | 4 | 4 |
| (Euchelata, Sars)— | |||||||||
| Nymphonidae | Large, fixed | Large, scape 1–jointed | 5 (7) | 8–10 ♂, ♀ | One row, serrate | 4–5 | Well segmented | 2, 3, 4 (5) | 1, 2, 3, 4 (5) |
| Pallenidae | „ | „ | 0 or rudimentary | 10 ♂, ♀ | „ | 4 | „ | (1, 2), 3, 4 | „ |
| Phoxichilidiidae | „ | „ | 0 | 5–6 ♂ | One row, simple | 4 | „ | 1, 2, 3, 4 | „ |
| (Achelata, Sars)— | |||||||||
| Phoxichilidae | Large, fixed | 0 | 0 | 7 ♂ | Scattered, simple | 4 | Well segmented | 2, 3, 4 | 1, 2, 3, 4 |
| Pycnogonidae | „ | 0 | 0 | 9 ♂ | Small, irregular | 4 | Segmented, condensed | 4 | 4 |
CLASS PYCNOGONIDA.[429]
Marine Arthropoda, with typically seven (and very exceptionally eight) pairs of appendages, of which none have their basal joints subservient to mastication, the first three are subject to suppression, the first (when present) are chelate, the second palpiform, the third ovigerous, and the rest form ambulatory limbs, usually very slender and long; with a suctorial proboscis, a limbless, unsegmented abdomen, and no manifest respiratory organs.

Fig. 282.—Decolopoda australis, Eights. A, × 1: from a specimen obtained at the South Shetlands by the Scotia Expedition. B, First appendage, or chelophore. (A, original; B, after Hodgson.)
Fam. 1. Decolopodidae.—Appendage I. dwarfed, but complete and chelate, scape with two joints; II. 9–10–jointed; III. well developed in both sexes, 10–jointed, the terminal joints with 532about four rows of teeth; five pairs of legs, destitute of accessory claws; genital apertures on all the legs (Bouvier).
Decolopoda australis, Eights[430] (1834), a remarkable form from the South Shetlands, recently re-discovered by the Scotia expedition. The animal is large, seven inches or more in total span, in colour scarlet; it was found in abundance in shallow water and cast upon the shore. The body is greatly condensed, the proboscis is “clavate, arcuated downwards,” and beset with small spines. A second Antarctic species, D. antarctica, has been described by Bouvier. The presence of a fifth pair of legs distinguishes Decolopoda from all known Pycnogons, except Pentanymphon. Stebbing would ally Decolopoda with, or even include it in, the Nymphonidae; but the presence of a second joint in the chelophoral scape, the number of joints in, and the armature on, the ovigerous legs, and the deflexed proboscis, are all characters either agreeing with or tending towards those of the Eurycididae; while the Colossendeidae would be very like Decolopoda were it not for the complete suppression of the chelophores. It seems convenient to constitute a new family for this remarkable form.
Fam. 2. Colossendeidae (Pasithoidae, Sars).—Appendage I. absent in adult; appendage II. very long, 10–jointed; appendage III. 10–jointed, clawed, with many rows of teeth; auxiliary claws absent; segments of trunk fused; proboscis very large, somewhat mobile; genital apertures, in at least some cases, on all the legs.
Pasithoe, Goodsir (1842), which Sars assumes as the type of the family, is here relegated to Ammothea.[431] Colossendeis, Jarszynsky (1870) (Anomorhynchus, Miers (1881), Rhopalorhynchus, Wood-Mason (1873)), remains as the only genus commonly accepted: large, more or less slender short-necked forms; world-wide, principally Arctic, Antarctic, and deep-sea; about twenty-five species.[432] The largest species, C. gigas, Hoek, from great depths 533in the Southern Ocean, has a span of about two feet. The North Atlantic C. proboscidea and Antarctic C. australis are very closely related to one another. Carpenter would retain the genus Rhopalorhynchus for R. kröyeri, W.-M. (Andamans), R. clavipes, Carp. (Torres Straits), and R. tenuissimus, Haswell (Australia), all more or less shallow-water species, excessively attenuated, with the second and third body-segments elongated, the caudal segment excessively reduced, the club-shaped proboscis on a slender stalk, and other common characters. Pipetta weberi, Loman (1904), is a large and remarkable form from the Banda Sea, apparently referable, in spite of certain abnormal features, to this family; the proboscis is extraordinarily long and slender; the palps have eight joints, the ovigerous legs eleven.
Fam. 3. Eurycididae (Ascorhynchidae, Meinert).—Appendage I. more or less reduced; appendage II. 10–jointed (absent in Hannonia); appendage III. 10–jointed, clawed, with more than one tow of serrated teeth; proboscis movably articulated and more or less bent under the body; auxiliary claws absent.

Fig. 283.—Eurycide hispida, Kr.; side view.
Eurycide, Schiödte (1857) (Zetes, Kröyer, 1845): Appendage I. with two-jointed scape, without chelae in adult; one species (E. hispida, (Kr.)), from the North Atlantic and Arctic, and two others from the East Indies, recently described by Loman. Barana arenicola, Dohrn (1881), is nearly allied. Ascorhynchus, G. O. Sars (1876) (Gnamptorhynchus, Böhm, 1879; Scaeorhynchus, Wilson, 1881), very similar to Eurycide, with which, according to Schimkewitsch, it should be merged, includes large, smooth, elongated forms, with long neck and expanded frontal region, and a long proboscis lacking the long scape that supports the proboscis in Eurycide; about twelve species, world-wide, mostly deep-water. Barana castelli, Dohrn, from Naples is akin to the foregoing genera, but seems to deserve generic separation from B. arenicola. Ammothea longicollis, Haswell, from Australia, is, as Schimkewitsch has already remarked, almost certainly a Eurycide, as is also, probably, Parazetes auchenicus, Slater, from Japan.
Hannonia typica, Hoek (1880), from Cape Town, is a remarkable form, lately redescribed by Loman. The chelophores are much reduced, the palps are absent; the ovigerous legs are 53410–jointed, and clawed; the terminal joints of the latter bear long straight spines, scattered over their whole surface; the proboscis is borne on a narrow stalk, and sharply deflexed. The eggs form a single flattened mass, as in Pycnogonum. While the lack of palps would set this genus among the Pallenidae, the remarkable proboscis seems to be better evidence of affinity with Ascorhynchus and Eurycide.[433]
Nymphopsis, Haswell (1881), is a genus of doubtful affinities, placed here by Schimkewitsch. The first appendage is well-developed and chelate; the palps are 9–jointed, the ovigerous legs are 7–jointed, none of the joints being provided with the compound spines seen in Nymphon and Pallene. It is perhaps an immature form. Schimkewitsch has described another species, N. korotnevi, and Loman a third, N. muscosus, both from the East Indies.
Fam. 4. Ammotheidae.—Akin to Eurycididae in having the proboscis more or less movably jointed to the cephalic segment, and appendage I. reduced, non-chelate in the adult; the body is compact and more or less imperfectly segmented; appendage II. 4–9–jointed; appendage III. clawless, and the number of joints sometimes diminished, with a sparse row of serrated spines; auxiliary claws usually present.
Ammothea, Leach (1815) (including Achelia, Hodge (1864) = the old non-chelate individuals): appendage I. very small, 2–jointed; appendage II. 8–9–jointed; caudal segment fused with last body-segment; about eighteen species, four from the South Seas, two or three from the East Indies, the rest mostly Mediterranean and North Atlantic, in need of revision. Ammothea longipes, Hodge, is the young of Achelia hispida, Hodge; and Ammothea magnirostris, Dohrn, is apparently the same species. A. fibulifera, Dohrn, seems identical with Achelia echinata, Hodge (of which A. brevipes, Hodge, is the young), and so probably is A. achelioides, Wilson; Endeis didactyla, Philippi (1843), is very probably the same species. A. uniunguiculata, Dohrn (? Pariboea spinipalpis, Philippi (1843)), has no auxiliary claws. Leionymphon, Möbius (1902), contains nine Antarctic forms, allied to Ammothea (including A. grandis, Pfeffer, and Colossendeis gibbosa, Möb., which two are probably identical), with characteristic 535transverse ridges on the body, a large proboscis, a 9–jointed palp, and somewhat peculiar ovigerous legs. Cilunculus, Fragilia, and Scipiolus are new genera more or less allied to Leionymphon, described by Loman (1908) from the Siboga Expedition.[434] Tanystylum, Miers (1879) (including Clotenia, Dohrn (1881), and Discoarachne, Hoek (1880)), has appendage I. reduced to a single joint or a small tubercle, and appendage II. 4–6–jointed; world-wide; about eight species. Austrodecus glacialis and Austroraptus polaris are two allied Antarctic species, described by Hodgson (1907), the former a curious little form with a pointed, weevil-like proboscis, no chelophores, and 6–jointed palp. Trygaeus communis, Dohrn (1881), from Naples, has a 7–jointed, and Oorhynchus aucklandiae, Hoek (1881), a 9–jointed palp; the former has only seven joints in the ovigerous leg. Lecythorhynchus armatus, Böhm (1879), with rudimentary 2–jointed chelophores, and L. (Corniger) hilgendorfi, Böhm, with small tubercles in their place, both from Japan, have also 9–jointed palps: the former, at least, is apparently an Ammothea. Several insufficiently described genera, Phanodemus, Costa (1836), Platychelus, Costa (1861), Oiceobathes, Hesse (1867), and Böhmia, Hoek (1880), seem to be referable to this group; all have chelate mandibles, and may possibly be based on immature forms.
Goodsir’s Pasithoe vesiculosa[435] is, in my opinion, undoubtedly Ammothea hispida, Hodge, and so also, I believe, is his Pephredo hirsuta; P. umbonata, Gould[436] (Long Island Sound), is, with as little doubt, Tanystylum orbiculare, Wilson.
Fam. 5. Rhynchothoracidae.—The animal identified by Dohrn as Rhynchothorax mediterraneus, Costa (1861), is a minute and very remarkable form, without chelophores, with large 8–jointed palps, reduced by fusion to five joints, and 10–jointed, clawed ovigerous legs, which last are provided on the last five joints with peculiar toothed tubercles. The general aspect of the body is somewhat like that of an Ammothea, which genus it resembles in the ventral insertion of the ovigerous legs and the somewhat imperfect segmentation of the body. It 536differs from Ammotheidae in the possession of a claw on appendage III. It is highly peculiar in the structure of the mouth, in having a long forward extension of the oculiferous tubercle jutting out over the proboscis, in the extreme shortness of the intestinal caeca and ovaries which scarcely extend into the legs, and in the absence of cement-glands from the fourth joint of the legs; these last are present only in the third joint of the penultimate legs. A single pair of generative orifices are found on the last legs. A second species, R. australis, Hodgson, comes from the Antarctic.

Fig. 284.—Rhynchothorax mediterraneus, Costa. A, Body and bases of legs; B, terminal joints of palp. (After Dohrn.)
Fam. 6. Nymphonidae.—Appendage I. well-developed, chelate; II. well-developed, usually 5–jointed; III. well-developed in both sexes, usually 10–jointed, the terminal joints with one row of denticulated spines.
Nymphon, Fabr. (1794), about forty-five recognised species, of which some are but narrowly defined. Closely allied are Chaetonymphon, G. O. Sars (1888), including thick-set, hairy species, about eight in number, from the North Atlantic, Arctic, and Antarctic; and Boreonymphon, G. O. Sars (1888), with one species (B. robustum, Bell, Fig. 276), also northern, in which the auxiliary claws are almost absent. Nymphon brevicaudatum, 537Miers (= N. horridum, Böhm), an extraordinary hispid form from Kerguelen,[437] is also peculiar. Pentanymphon, Hodgson (1904), from the Antarctic (circumpolar), differs in no respect save in the presence of a fifth pair of legs; one species.
The only other genus is Paranymphon, Caullery (1896) (one species, Gulf of Gascony, West of Ireland, Greenland), in which the palp is (6–)7–jointed, the ovigerous leg 8–jointed, and the auxiliary claws are absent.
Fam. 7. Pallenidae.—As in Nymphon, but appendage II. absent or rudimentary.

Fig. 285.—Pallene brevirostris, Johnston, ♀, Plymouth.
Pallene, Johnston (1837): about ten species (Mediterranean, North Atlantic, Arctic, Australia). P. languida, Hoek, Australia, lacks auxiliary claws, and is otherwise distinct; but P. novaezealandiae, G. M. Thomson, is typical. Pseudopallene, Wilson (1878):[438] appendage III. clawed; auxiliary claws absent; four (or more) species (North Atlantic, Arctic, Antarctic). P. (Phoxichilus) pygmaea, Costa (1836), and P. spinosa, Quatref., seem to belong to this genus or to Pallene. Cordylochele, G. O. Sars (1888): closely allied, but with front of cephalic segment much expanded and chelae remarkably swollen, includes three very smooth, elongated, northern species, to which Bouvier has added one from the Antarctic; Pallene laevis, Hoek, from Bass’s Straits, is somewhat similar. Neopallene, Dohrn (1881): as in Pallene, but with a rudimentary second appendage in the female, and no generative aperture on the last leg in the male (one species, Mediterranean). Parapallene, Carpenter (1892): as in Pallene, but without auxiliary claws, and with the two last segments of the trunk (which in Pallene are coalesced) independent (about 538ten species, East Indies and Australia); Pallene grubii, Hoek (Phoxichilidium sp., Grube, 1869), is probably congeneric. Pallenopsis, Wilson (1881): appendage I. 2–jointed; appendage II. rudimentary, 1–jointed; appendage III. clawless; auxiliary claws present; slender forms, including some formerly referred to Phoxichilidium; about fifteen species, world-wide. Pallene dimorpha, Hoek, from Kerguelen, with 4–jointed palps, deserves a new generic appellation. P. longiceps, Böhm, from Japan, with rudimentary 2–jointed palps in the male, is also peculiar.

Fig. 286.—Phoxichilidium femoratum, Rathke, Britain. A, The animal with its legs removed; B, leg and chela.
Fam. 8. Phoxichilidiidae.—Appendage I. well-developed; II. absent; III. present only in the male, having a few simple spines in a single row. The last character is conveniently diagnostic, but nevertheless the Phoxichilidiidae come very near to the Pallenidae, with which, according to Schimkewitsch and others, they should be merged; the two families resemble one another in the single row of spines on the ovigerous legs and in the extension of the cephalic segment over the base of the proboscis.
Phoxichilidium, M.-E. (1840): appendage III. 5–jointed; five or six species (Mediterranean, North Atlantic, Arctic, Australia, Japan). Anoplodactylus, Wilson (1878): appendage III. 6–jointed; auxiliary claws absent or very rudimentary; about twelve species, cosmopolitan, of which many were first 539referred to Phoxichilidium. A. neglectus, Hoek, comes from 1600 fathoms off the Crozets. Oomerus stigmatophorus, Hesse (1874), from Brest, seems to belong to one or other genus, but is unrecognisable. Anaphia, Say (1821), is in all probability identical with Anoplodactylus, and if so the name should have priority. Halosoma, Cole (1904), is an allied genus from California.

Fig. 287.—Anoplodactylus petiolatus, Kr., Britain. A, Dorsal view; B, side view.
Fam. 9. Phoxichilidae.[439]—Appendage I. and II. absent; appendage III. present only in the males, 7–jointed, with minute scattered spines; auxiliary claws well-developed; body and legs slender. The only genus is Phoxichilus (auctt., non Latreille, Chilophoxus, Stebbing, 1902); the type is P. spinosus, Mont. (non Quatrefages), from the N. Atlantic, and P. vulgaris, Dohrn, P. charybdaeus, Dohrn, and P. laevis, Grube, are all very similar. Endeis gracilis, Philippi (1843), is probably identical with P. spinosus, or one of its close allies. There are also known P. meridionalis, Böhm, P. mollis, Carp., and P. procerus, Loman, from the East Indies; P. australis, Hodgson, from the Antarctic; P. böhmii, Schimk., of unknown locality; and forms ascribed to P. charybdaeus by Haswell and by Schimkewitsch from Australia and Brazil.
Fam. 10. Pycnogonidae.—Appendages I. and II. absent; appendage III. present only in the male, 9–jointed, with small, simple spines; auxiliary claws absent or rudimentary; body and legs short, thick-set.
The only genus is Pycnogonum, Brünnich (1764) (Polygonopus, 540Pallas, 1766); the type is P. littorale, Ström, of the N. Atlantic (0–430 fathoms), to which species have also been ascribed forms from various remote localities, e.g. Japan, Chile, and Kerguelen. P. crassirostre, G. O. Sars, a northern and more or less deep-sea form, is distinct, and so also are P. nodulosum and P. pusillum, Dohrn, from Naples. P. stearnsi, Ives, from California, is like P. littorale, except for the rostrum, which resembles that of P. crassirostre. P. magellanicum, Hoek, P. magnirostre, Möbius, both from the Southern Ocean; P. microps, Loman, from Natal, and four others described by Loman from the East Indies, are the other authenticated species. Of P. philippinense, Semper, I know only the bare record; and P. australe, Grube, is described only from a larval form with three pairs of legs. P. orientale, Dana (first described as Astridium, n.g.), is also described from an immature specimen, and more resembles a Phoxichilus.
The British Pycnogons.
Dr. George Johnston,[440] the naturalist-physician of Berwick-on-Tweed, Harry Goodsir,[441] brother of the great anatomist, who perished with Sir John Franklin, and George Hodge[442] of Seaham Harbour, a young naturalist of singular promise, dead ere his prime, were in former days the chief students of the British Pycnogons. Of late, Carpenter[443] has studied the Irish species; and the cruises of the Porcupine, Triton, and Knight Errant have given us a number of deep-water species from the verge of the British area.
In compiling the following list, I have had the indispensable advantage of access to Canon Norman’s collection, and the still greater benefit of his own stores of endless information.[444]
Pseudopallene circularis, Goodsir: Firth of Forth.
Phoxichilidium femoratum, Rathke (P. globosum, Goodsir; Orithyia coccinea, Johnston) (Figs. 270, B; 286): East and West coasts, Shetland, Ireland.
Anoplodactylus virescens, Hodge (? Phoxichilidium olivaceum, Gosse): South coast.
541A. petiolatus, Kr. (Figs. 270, C; 275, B; 287) (Pallene attenuata and pygmaea, Hodge; Phoxichilidium exiguum and longicolle, Dohrn): Plymouth, Firth of Forth, Cumbrae, Irish coasts.
Ammothea (Achelia) echinata, Hodge (Fig. 265, B; 274, 4; 275, E): Plymouth, Channel Islands, Isle of Man, Cumbrae, Durham (Hodge), West of Ireland. We have not found it on the East of Scotland. A. brevipes, Hodge, is presumed to be the young. Two of Dohrn’s Neapolitan species, A. fibulifera and A. franciscana, are in my opinion not to be distinguished from one another, nor from the present species.
A. hispida, Hodge (Fig. 266, C) (A. longipes, Hodge (juv); A. magnirostris, Dohrn;? Pasithoe vesiculosa, Goodsir;? Pephredo hirsuta, Goodsir): Cornwall and Devon (Hodge and Norman), Jersey. The form common on the East of Scotland would seem to be this species. The Mediterranean A. magnirostris, Dohrn, appears to be identical.
A. laevis, Hodge: Cornwall (Hodge), Devon (Norman), Jersey (Sinel).
Tanystylum orbiculare, Wilson (Clotenia conirostre, Dohrn): Donegal (Carpenter).
Phoxichilus spinosus, Mont. (Fig. 265, C; 270, A; 275, C): South Coast, Moray Firth, Firth of Clyde, Ireland. A smaller and less spiny form occurs, which Carpenter records as P. laevis, Grube, but Norman unites the two under the name of Endeis spinosus (Mont.).
Pycnogonum littorale, Ström (Fig. 262): on all coasts, and to considerable depths (150 fathoms, West of Ireland).
Nymphon brevirostre, Hodge (N. gracile, Sars) (Figs. 263, 264, 267, A; 272, 274, 3): common on the East Coast; Herm (Hodge), Dublin, Queenstown (Carpenter). Our smallest species of Nymphon.
N. rubrum, Hodge (N. gracile, Johnston; N. rubrum, G. O. Sars): common on the East Coast; Oban (Norman), Ireland (Carpenter).
N. grossipes, O. Fabr., Johnston (N. johnstoni, Goodsir): Northumberland, East of Scotland, Orkney, etc., not uncommon.
N. gracile, Leach (N. gallicum, Hoek; ♂ N. femoratum, Leach): South of England, West of Scotland, and Ireland.
N. strömii, Kr. (N. giganteum, Goodsir) (Figs. 273, 274, 2): East Coast, from Holy Island to Shetland.
Chaetonymphon hirtum, Fabr. (Fig. 274, 1): Northumberland (Hodge), Margate (Hoek), East of Scotland, and Ireland, not uncommon. There seems to be no doubt that British specimens agree with this species as figured and identified by Sars. N. spinosum, Goodsir (East of Scotland, Goodsir; Belfast, W. Thompson), is, according to Norman, the same species. Sars’ Norwegian specimens figured under the latter name are not identical, and have been renamed by Norman C. spinosissimum, but are said by Meinert and Möbius to be identical with C. hirtipes, Bell.
Hodge (1864) records Nymphon mixtum, Kr., and N. longitarse, Kr., from the Durham coast. His full list of the recorded species of other authors also includes the following doubtful or unrecognised species: N. pellucidum, N. simile, and N. minutum, all of Goodsir.
Pallene brevirostris, Johnston (P. empusa, Wilson;? P. emaciata, Dohrn) (Figs. 275, A; 285): all coasts. Examples differ considerably in size and proportions, as do Dohrn’s Neapolitan species one from another. We have specimens from the Sound of Mull that come very near, and perhaps agree 542with, Sars’ P. producta, a species that scarcely differs from P. brevirostris, save in its greater attenuation; the same species has also been recorded from Millport and from Port Erin.
P. spectrum, Dohrn: Plymouth (A. H. Norman).
Besides the above, all of which are littoral or more or less shallow-water species, we have another series of forms, or, to speak more correctly, we have two other series of forms, from the deep Atlantic waters within the British area. In the cold area of the Faeroe Channel we have Boreonymphon robustum, Bell; Nymphon elegans, Hansen; N. sluiteri, Hoek; N. stenocheir, Norman; Colossendeis proboscidea, Sabine; C. angusta, Sars. In the warm waters south and west of the Wyville-Thomson ridge we have Chaetonymphon spinosissimum, Norman; Nymphon gracilipes, Heller (non Fabr.); N. hirtipes, Bell; N. longitarse, Kr.; N. macrum, Wilson; Pallenopsis tritonis, Hoek (= P. holti, Carpenter); Anoplodactylus oculatus, Carpenter, and A. typhlops, G. O. Sars; and to the list under this section Canon Norman has lately made the very interesting addition of Paranymphon spinosum, Caullery, from the Porcupine Station XVII., S.S.E. of Rockall, in 1230 fathoms. Lastly, and less clearly related to temperature, we have Chaetonymphon tenellum, Sars; N. gracilipes, Fabr.; N. leptocheles, Sars; N. macronyx, Sars; N. serratum, Sars; and Cordylochele malleolata, Sars.
Of the species recorded in the above list as a whole, Anoplodactylus virescens, Nymphon gracile, and Pallene spectrum reach their northern limit in the southern parts of our own area; Ammothea echinata, Anoplodactylus petiolatus, Pallene brevirostris, and Phoxichilus spinosus (or very closely related forms) range from the Mediterranean to Norway, the last three also to the other side of the Atlantic; Nymphon brevirostre and N. rubrum range from Britain, where they are in the main East Coast species, to Norway. Of the Atlantic species, other than the Arctic ones, the majority are known to extend to the New England coast.
INDEX
Every reference is to the page: words in italics are names of genera or species; figures in italics indicate that the reference relates to systematic position; figures in thick type refer to an illustration; f. = and in following page or pages; n. = note.
- Abalius, 312
- Abdomen, of Malacostraca, 110;
- of Acantholithus, 178;
- of Birgus, 176;
- of Cenobita, 176;
- of Dermaturus, 178;
- of Hapalogaster, 178;
- of Lithodes, 178;
- of Pylopagurus, 178;
- of Trilobites, 235;
- of Scorpions, 297;
- of Pedipalpi, 309;
- of Spiders, 317;
- of Palpigradi, 422;
- of Solifugae, 426;
- of Pseudoscorpions, 431;
- of Podogona, 440;
- of Phalangidea, 440, 443;
- of Acarina, 457;
- of Pentastomida, 489;
- of Pycnogonida, 502
- Abdominal glands, of Chernetidea, 432
- Abyssal region (marine), 204;
- (lacustrine), 209
- Acantheis, 418
- Acanthephyra, 163
- Acanthephyridae, 163
- Acanthoctenus, 415
- Acanthodon, 388
- Acanthogammarus, 138
- Acantholeberis, 53
- Acantholithus, 181;
- A. hystrix, 178
- Acanthophrynus, 313
- Acari, 454 (= Acarina, q.v.)
- Acaridea, 454 (= Acarina, q.v.)
- Acarina, 258, 454 f.;
- Acaste, 249
- Accola, 390
- Acerocare, 247
- Achelata, 529
- Achelia, 534;
- A. longipes, 506
- Achtheres, 75;
- A. percarum, 75
- Acidaspidae, 251
- Acidaspis, 226, 227, 230, 231, 235, 241, 251;
- Aciniform glands, 335, 349
- Acoloides saitidis, 367
- Acroperus, 53;
- A. leucocephalus, 52
- Acrosoma, 410
- Acrothoracica, 92
- Actaea, 191;
- habitat, 198
- Actinopodinae, 387
- Actinopus, 387
- Aculeus, of scorpion, 303
- Admetus, 313
- Aegidae, 126
- Aegisthus, 61
- Aeglea laevis, 169;
- distribution, 212
- Aegleidae, 169
- Aeglina, 227, 249;
- Ae. prisca, 248
- Agelena, 416;
- Agelenidae, 325, 352, 353, 415
- Ageleninae, 416
- Aggregate glands, 335, 349
- Aglaspis, 279
- Agnathaner, 66
- Agnathonia, 529
- Agnostidae, 244
- Agnostini, 243
- Agnostus, 222, 223, 225, 231, 234, 245;
- A. integer, 245
- Agraulos, 247
- Agroeca, 397;
- A. brunnea, cocoon, 358
- Albunea, 171;
- Albuneidae, 171
- Alcippe, 92;
- Alcock, on Oxyrhyncha, 192;
- on phosphorescence, 151
- Alepas, 89
- Alima, larva of Squilla, 143
- Alimentary canal, of Crustacea, 14;
- 544Trilobites, 222;
- Alitropus (Aegidae), habitat, 211
- Allman, on larvae of Pycnogons, 523
- Alloptes, 466
- Alona (including Leydigia, Alona, Harporhynchus, Graptoleberis), 53
- Alonopsis, 53
- Alpheidae, 163;
- habitat, 198
- Alpheus, 163;
- reversal of regeneration, 156
- Alveolus, of palpal organ of Spiders, 322
- Amaurobius, 399;
- Amblyocarenum, 388
- Amblyomma, 470;
- Amblypygi, 312
- Ammothea, 505, 534;
- A. achelioides, 534;
- A. brevipes, 541;
- A. echinata, 505, 509, 510, 534, 541, 542;
- A. fibulifera, 522, 534, 541;
- A. franciscana, 541;
- A. grandis, 534;
- A. hispida, 534, 535, 541;
- A. laevis, 541;
- A. longicollis, 533;
- A. longipes, 506, 534, 541;
- A. magnirostris, 534, 541;
- A. typhlops, 542;
- A. uniunguiculata, 534
- Ammotheidae, 534
- Amopaum, 452
- Ampharthrandria, 61
- Amphascandria, 57
- Amphion, 251
- Amphipoda, 136 f.;
- Ampullaceal glands, 335, 349
- Ampycini, 243
- Ampyx, 231, 245;
- A. roualti, 230
- Anabiosis, in Tardigrada, 484
- Analges, 455, 466
- Analgesinae, 466
- Ananteris, 306
- Anaphia, 539
- Anaspidacea, 115;
- Anaspidae, 89
- Anaspides, 115, 117;
- Anaspididae, 115
- Anelasma squalicola, 89
- Anelasmocephalus, 452
- Angelina, 247
- Anisaspis bacillifera, 387
- Anisopoda, 122
- Anomalocera pattersoni, 60;
- Anomopoda, 51
- Anomorhynchus, 532
- Anomura, 167;
- relation to Thalassinidea, 167
- Anoplodactylus, 511, 538;
- Anopolenus, 247
- Antarctic zone (marine), 200
- Antarctica, evidence on, 200, 217
- Antennae, of Crustacea, 5, 8;
- Antennary gland, 13 (= green gland, q.v.)
- Anthrobia, 406;
- Anthura, 124
- Anthuridae, 124
- Ants and spiders, 370
- Anyphaena accentuata, 397
- Aphantochilinae, 414
- Aphantochilus, 414
- Apoda, 94
- Apodidae, 19, 21, 22, 23, 27, 28, 29, 31, 36, 241
- Aponomma, 470
- Appendages (incl. legs, limbs), of Crustacea, 7;
- of Entomostraca, 18;
- of Phyllopoda, 24;
- of Cladocera, 40;
- of Copepoda, 55;
- of Cirripedia, 80 f.;
- of Ostracoda, 107;
- of Malacostraca, 110;
- of Nebalia, 111;
- of Eumalacostraca, 113;
- of Anaspides, 115;
- of Mysidacea, 118 f.;
- of Cumacea, 120;
- of Isopoda, 121 f.;
- of Amphipoda, 136 f.;
- of Stomatopoda, 142;
- of Euphausiacea, 144 f.;
- of Decapoda, 152;
- of Macrura, 153;
- of their larvae, 159;
- of Anomura, 167 f.;
- of Birgus, 175;
- of Brachyura, 181 f.;
- alterations caused by parasites, 100 f.;
- by hermaphroditism, 102 f.;
- of Trilobita, 236, 237;
- of Arachnida, 255 f.;
- of Limulus, 262, 263;
- of Eurypterus, 285 f.;
- of Scorpions, 301, 303;
- of Pedipalpi, 309;
- of Spiders, 319;
- of Palpigradi, 422;
- of Solifugae, 426;
- of Pseudoscorpions, 432;
- of Podogona, 440;
- of Phalangidea, 443;
- of Acarina, 458;
- of Tardigrada, 479;
- of Pentastomida, 493;
- of Pycnogons, 503 f.
- Apseudes spinosus, 123
- Apseudidae, 122
- Apstein, 335
- Apus, 21, 23, 25, 28, 30, 32, 34, 36, 221, 242, 243;
- Arachnida, introduction to, 255;
- Araneae, 258, 314 f.
- Araneida, 314
- Araneina, 314
- Araneus, 408 n.
- Aratus pisonii, 195
- Arbanitis, 388
- Archaeolepas, 84;
- A. redtenbacheri, 84
- Archea, 411;
- Archeidae, 321, 411
- Archisometrus, 306
- Arctic zone, 199
- Arcturidae, 127
- Arcturus, 127
- Arcyinae, 410
- Arcys, 410
- Arethusina, 223, 230, 251;
- A. konincki, 250
- Argas, 457, 469;
- Argasidae, 469
- Arges, 252
- Argiope, 408;
- Argiopidae, 406 n.
- Argiopinae, 408
- Argulidae, 76
- Argulus foliaceus, 77
- Argyrodes, 402;
- Argyrodinae, 402
- Argyroneta, 336, 415;
- Ariadna, 395
- Ariamnes, 402;
- A. flagellum, 318
- Arionellus, 247
- Aristaeus, 162;
- Armadillidium, 129
- Artema, 401
- Artemia, 23, 24, 35;
- Arthrolycosa antiqua, 383
- Arthropoda, 4;
- Arthrostraca, 121
- Asagena, 404
- Asaphellus, 249
- Asaphidae, 249
- Asaphini, 243
- Asaphus, 222, 225, 227, 229, 235, 236, 249;
- Ascidicola rosea, 66
- Ascidicolidae, 66
- Asconiscidae, 130
- Ascorhynchus, 505, 533;
- Ascothoracica, 93
- Asellidae, 128
- Asellota, 127
- Asellus, 127;
- Aspidoecia, 76
- Astacidae, 157;
- Astacoides, 157;
- distribution, 213
- Astacopsis, 157;
- Astacus, 104, 157;
- Astacus gammarus (= Homarus vulgaris), 154
- Asterocheres violaceus, 67
- Asterocheridae, 67
- Asterope oblonga, 108
- Astia, 421;
- A. vittata, 381
- Astigmata, 465
- Astridium, 540
- Atax, 462, 472;
- Atelecyclidae, 190
- Atelecyclus, 191;
- respiration, 189
- Atops, 247
- Attidae, 376, 381, 419
- Attus, 421;
- Atya, 163
- Atyephyra, 163;
- habitat, 210
- Atyidae, 159, 163;
- distribution, 212
- Atypidae, 390
- Atypoides, 391
- Atypus, 391;
- Auditory organ, of Anaspides, 116;
- Augaptilus filigerus, 59
- Austrodecus glacialis, 535
- Austroraptus polaris, 535
- Autotomy, 155
- Avicularia, 389
- Aviculariidae, 316, 327, 386;
- Aviculariinae, 389
- Axial furrows, 223
- Baglivi, 361
- Baikal, Lake, Crustacea of, 212
- Balanus, 91;
- Ballus variegatus, 420
- Barana, 506, 513, 533;
- Barnacles, origin of term, 79
- Barrande, J., on development of Trilobites, 238;
- on their classification, 243
- Barrandia, 249
- Barrois, 435 n.
- 546Barrus, 429
- Barychelinae, 389
- Basse, on Tardigrada, 481
- Baster, Job, 503
- Bates, 373
- Bathynomus giganteus, 126;
- habitat, 205
- Bathynotus, 247
- Bathyphantes, 406
- Bdella lignicola, 471
- Bdellidae, 458, 471
- Beecher, C. E., on facial sutures of Agnostus and Olenellus, 225;
- Beetle-mites, 467
- Beetle-parasites, 470
- Belinurus, 275, 279;
- B. reginae, 278
- Belisarius, 308
- Belt, 368, 371
- Beltina, 283 n.
- Bernard, 311, 424, 426, 433 n., 434 n.
- Bertkau, 323, 365, 395 n.
- Beyrich, E., on facial suture of Trinucleus, 226
- Billings, E., on appendages of Trilobites, 236
- Bipolarity, 200
- Birds and Spiders, 370
- Birds’ feather Mites, 466
- Birgus, 181;
- Black Corals, Cirripedia parasitic on, 93, 94
- Blackwall, 348, 359 n., 365, 368, 385
- Blindness, in Crustacea, 149, 209, 210;
- in Spiders, 334
- Blood, haemoglobin supposed in, 30, 68
- Boas, on classification of Malacostraca, 113
- Boeckella, distribution, 216
- Boeckia, 138
- Böhmia, 535
- Bolocera, Pycnogonum with, 524
- Bolyphantes, 406
- Bomolochidae, 71
- Bomolochus, 71, 72
- Bon, 360
- Bont-tick, 456
- Boophilus, 456, 469;
- B. australis, capitulum of, 468
- Bopyridae, 130, 133
- Bopyrina, 129, 130, 132
- Bopyrus fougerouxi, 133;
- Bopyrus larva, of Bopyrina, 129, 133
- Boreomysis, 120;
- B. scyphops, distribution, 201
- Boreonymphon, 536;
- Bosmina, 52, 53;
- Bosminidae, 53;
- Bothriuridae, 306, 308
- Bothriurus, 308
- Bouvier, 528 n.
- Boys, 348, 360, 376
- Brachybothrium, 391
- Brachymetopus, 251
- Brachythele, 390
- Brachyura, 181;
- eyes, 150
- Branchiae (= gills) of Crustacea, 16;
- Branchinecta, 25, 35;
- Branchiopoda, 18 f.
- Branchiopodopsis, 35;
- B. hodgsoni, 35
- Branchiostegite, 152
- Branchipodidae, 19, 22, 35, 241
- Branchipus, 25, 35, 233, 242, 511 n.;
- Branchiura, 76
- Brauer, on development of Scorpions, 263, 301 n., 305
- Breeding (see Reproduction)
- British forms, of Cladocera, 51;
- of Pycnogons, 540
- Bronteidae, 249
- Bronteus, 228, 235, 249;
- Brood-pouch, of Cladocera, 46, 47;
- of Peracarida, 118
- Broteas, 308
- Broteochactas, 308
- Brünnich, 502
- Buckler, 330
- Bucranium, 414
- Bulb, of palpal organ of Spiders, 322
- Bumastus, 235, 236, 249
- Bunodella, 279
- Bunodes, 279
- Buthidae, 306
- Buthinae, 306
- Buthus, 306;
- Bythotrephes, 38, 54;
- Cabiropsidae, 130
- Caecidotea nickajackensis, habitat, 210;
- C. stygia, habitat, 210
- Caeculinae, 472
- Caeculus, 472
- Calamistrum, 326, 354, 385, 392, 399, 410
- Calanidae, 57
- Calanus, 57;
- Calappa, 187;
- 547Calappidae, 187
- Calathocratus, 452
- Calathura brachiata (Anthuridae), Duplorbis parasitic on, 95
- Calicurgus annulatus, 369
- Caligidae, 73
- Caligus nanus, 74;
- Callianassa, 167;
- Callianassidae, 167
- Callinectes, 191;
- C. sapidus, 191
- Calman, on classification of Crustacea, 112, 113
- Calocalanus plumulosus, 58
- Caloctenus, 418
- Calommata, 391
- Calymene, 225, 230, 235, 249;
- Calymenidae, 247
- Calyptomera, 38, 51
- Calyptopis, larva of Euphausia pellucida, 144
- Cambaroides, distribution, 213
- Cambarus, 157;
- Camerostome, 452
- Campbell, 327
- Camptocercus, 53;
- C. macrurus, 48
- Cancer, 191;
- C. pagurus, 191
- Cancerilla, 68;
- C. tubulata, 68
- Cancridae, 191
- Candace, 60;
- C. pectinata, 60
- Candacidae, 60
- Candona, 107;
- C. reptans, 107
- Canestrini, 464
- Canthocamptus, 62;
- habitat, 206
- Capitulum, of Cirripedia, 81;
- Caponia natalensis, 395
- Caponiidae, 395
- Caponina, 395
- Caprella acutifrons, 140;
- C. grandimana, 139
- Caprellidae, 139
- Carapace, of Phyllopoda, 19 f.;
- Carcinoplacidae, 195
- Carrinoscorpius, 277;
- C. rotundicauda, 277
- Carcinus, 191;
- Cardisoma, 196;
- distribution, 201
- Caridea, 158, 163;
- metamorphosis, 161
- Caridina, 163;
- C. nilotica, distribution, 212
- Carniola, caves of, 34
- Carpenter, on segmentation of Arthropods, 6, 263;
- Caruncle, 470
- Caspian Sea, Crustacea of, 215
- Caspiocuma, 121
- Catometopa, 193 f.;
- Catophragmus, 91
- Caudal organs, 311
- Caullery, on Liriopsidae, 132 n.
- Causard, 332
- Cavanna, 520
- Cecrops, 74
- Cenobita, 181;
- relation to Birgus, 176
- Cenobitidae, 181
- Centropages hamatus, 203;
- C. typicus, distribution, 203
- Centropagidae, 58
- Centropelma, 416
- Centropleura, 247
- Centrurinae, 306
- Centrurus, 306
- Cephalic shield, 223
- Cepheus ocellatus, 467
- Cerataspis, 162
- Ceratolichas, 252
- Ceratopyge, 247
- Cercophonius, 308
- Ceriodaphnia, 37, 39, 51
- Ceroma, 429
- Chactas, 308
- Chactidae, 306, 307
- Chaerilidae, 306, 307
- Chaerilus, 307
- Chaetolepas, 89
- Chaetonymphon, 536;
- Chaetopelma, 389
- Charontinae, 313
- Chasmops, 249
- Cheeks, of Trilobites, 223, 225
- Cheese-mites, 466
- Cheiracanthium, 397
- Cheiruridae, 250
- Cheirurus, 235, 251;
- Chelicerae, of Xiphosura, 263 f.;
- Chelifer, 436, 437;
- Chelifera, 122
- Cheliferidae, 436
- Chelophores, of Pycnogons, 505
- Chernes, 432, 436, 437, 438
- 548Chernetes, 430
- Chernetidea, 258, 430 f.
- Cheyletinae, 473
- Cheyletus, 458, 473
- Chilaria, 260, 271, 287, 292
- Chilobrachys, 390;
- Chilophoxus, 539
- Chiltonia, 139;
- distribution, 217
- Chiridium, 432, 436, 437;
- C. museorum, 437
- Chirocephalus, 35;
- Chlorodinus, habitat, 198
- Chlorodius, 191
- Chondracanthidae, 72
- Chondracanthus zei, 72
- Choniostoma, 76
- Choniostomatidae, 76
- Chthonius, 436, 438
- Chun, on phosphorescence and eyes, 150
- Chydorus, 54
- Cilunculus, 535
- Circulatory (= vascular) system, of Crustacea, 11;
- Cirolana, 126
- Cirripedia, 79 f.;
- Cladocera, 19, 37 f.;
- Claparède, 331, 462 n.
- Clarke, J. M., on the eye of Calymene senaria, 229;
- of Harpes, 231
- Claus, on Copepoda, 55;
- Claw-tufts, 389
- Clerck, 384, 408 n.
- Clibanarius, 181
- Clotenia conirostre, 541
- Clubiona, 337, 368, 397;
- Clubioninae, 397
- Clypeus, 316
- Clytemnestra, 61
- Coelotes atropos, 416
- Cole, 520, 524, 525, 528 n.
- Colossendeis, 505, 532;
- Colour, adaptation in, of Crustacea, 159
- Colulus, 317, 319
- Commensalism, of Hermit-crabs, 172;
- of Pinnotheres, 195
- Complemental males, of Cirripedes, 83, 86, 99, 106
- Conchoderma, 88;
- C. virgata, 88
- Conocephalidae, 247
- Conocoryphe, 231, 247;
- C. sulzeri, 248
- Conocoryphidae, 247
- Conolichas, 252
- Conothele, 388
- Constantia (Macrohectopus), 138;
- occurrence, 212
- Cook, 425 n.
- Copepoda, 55 f.;
- Copilia vitrea, 69, 70
- Cordylochele, 506, 537;
- Corniger hilgendorfi, 535
- Coronula diadema, 91
- Corophiidae, 139
- Corophium, 139
- Corycaeidae, 69
- Corystes, 188, 190;
- Corystidae, 190
- Cosmetidae, 449
- Costa, da, 221
- Coxal glands, 257;
- Coxopodite, of Trilobites, 237
- Crab, Hermit-, 171–173;
- Crab-spiders, 412 (= Thomisidae, q.v.)
- Crangon, 164;
- Crangonidae, 164;
- distribution, 199
- Crangonyx, 138
- Crayfish, 154, 157;
- Crevettina, 137
- Cribellatae, 324, 385, 386 n.
- Cribellum, 326, 354, 385, 386, 392, 398, 410
- Croneberg, 460
- Cruregens, 124;
- C. fontanus, habitat, 210
- Crustacea, organisation, 1 f.;
- segmentation, 5;
- appendages, 8 f.;
- body-cavity and coelom, 11;
- kidneys, 13;
- alimentary canal, 14;
- reproductive organs, 15;
- respiratory organs, 16;
- compound eyes, 146;
- growth and sex in, 100;
- metabolism, 104;
- distribution, 197;
- pelagic, 202, 207;
- littoral, 197, 206;
- abyssal, 204, 209;
- fresh-water, 205;
- subterranean and cave, 209
- Crustacés aranéiformes, 501 n.
- Cryphaeus, 249
- Cryphoeca, 416
- Cryptocellus, 439;
- C. simonis, 439
- 549Cryptocerus, 414
- Cryptoniscidae, 130
- Cryptoniscina, 129, 130
- Cryptoniscus, larva of Epicarida, 129, 131, 132
- Cryptophialus, 92;
- Cryptostemma westermannii, 439
- Cryptostemmatidae, 440
- Cryptothele, 400
- Ctenidae, 418
- Cteninae, 418
- Cteniza, 388;
- C. ariana, 355
- Ctenizinae, 388
- Ctenocephalus, 247
- Ctenophora, 412
- Ctenopoda, 51
- Ctenopyge, 232, 247
- Ctenus, 418
- Cucullus, 440
- Cuma, 121
- Cumacea, 114, 120;
- of the Caspian, 215
- Cumidae, 121
- Cyamidae, 140
- Cyamus ceti, 140
- Cybaeinae, 415
- Cybele, 251
- Cyclaspis, 121
- Cyclestheria, 37;
- C. hislopi, 37
- Cyclodorippe dromioides, eyes, 149
- Cyclograpsus, 196;
- distribution, 200
- Cyclometopa, 188 f.;
- Cyclopidae, 61, 62;
- subterranean, 209
- Cyclops, 62;
- Cyclosa conica, 409
- Cyclosternum, 389
- Cydrela, 399
- Cymodoce, 126
- Cymonomus, 188;
- Cymothoa, 126;
- habitat, 211
- Cymothoidae, 126
- Cyphaspis, 251
- Cyphophthalmi, 443, 444, 447
- Cypridae, 107;
- subterranean, 209
- Cypridinidae, 108
- Cypris, 107;
- C. reptans, parthenogenesis, 108
- Cypris larva, of Cirripedia, 80, 82;
- Cyrtauchenius, 388;
- C. elongatus, funnel of, 356
- Cythere dictyon, 108
- Cytherellidae, 109
- Cytheridae, 107
- Dactylopisthes digiticeps, 405
- Dactylopus tisboides, 62
- Daesia, 429
- Daesiinae, 429
- Dajidae, 130
- Dalmanites, 249;
- Danalia curvata, 130, 131, 132
- Daphnella, 51;
- testes, 44
- Daphnia, 37, 38, 39, 51;
- Daphniidae, 51;
- Darwin, on Cirripedia, 80, 85, 86, 92, 94
- Dasylobus, 450
- Decapoda, 152 f.;
- Dechenella, 251
- Decolopoda, 504, 529, 532;
- Decolopodidae, 531
- Defective orb-webs, 349
- Deiphon, 235, 251;
- D. forbesi, 250
- Delena, 414
- Delobranchiata, 258, 259 f.
- Demodex, 465;
- D. folliculorum, 465
- Demodicidae, 455, 465
- Dendrogaster astericola, 94
- Dermacentor, 469
- Dermanyssinae, 471
- Dermanyssus avium, 471
- Dermaturus, 181;
- D. hispidus, 178
- Desis, 415
- Deutovum, 462
- Development, of Monstrillidae, 64;
- of Cirripedia, 80;
- of Rhizocephala, 96;
- of Epicarida, 130;
- of Stomatopoda, 142;
- of Shrimps and Prawns, 159;
- of Loricata, 165;
- of Hermit-Crabs, 179;
- of Brachyura, 181;
- of Trilobites, 238 f.;
- of Limulus, 275;
- of Scorpio, 305;
- of Pseudoscorpions, 434;
- of Mites, 462;
- of Tardigrada, 483;
- of Pentastomida, 493;
- of Pycnogons, 520
- Diaea, 412;
- D. dorsata, 413
- Diaphragm, of Solifugae, 427
- Diaptomus, 59;
- Diastylidae, 121
- Diastylis, 121;
- Dichelaspis, 88
- Dichelestiidae, 68;
- classification, 63
- Dichelestium, 68
- Dick, 363
- Dicranogmus, 252
- Dicranolasma, 452
- Dictyna, 398;
- 550Dictynidae, 352, 353, 398
- Digestive system, = alimentary canal, q.v.
- Dikelocephalus, 247
- Dimorphism, high and low;
- Dindymene, 251
- Dinopinae, 410
- Dinopis, 410
- Dinorhax, 429
- Diogenes, 181
- Dionide, 245
- Diphascon, 485;
- Diplocentrinae, 306, 307
- Diplocentrus, 307
- Diplocephalus bicephalus, 405
- Diplostichous eyes, 301
- Diplura, 390
- Diplurinae, 390
- Dipoena, 403
- Discoarachne, 512, 535
- Distribution, of Crustacea, 197 f.;
- (stratigraphical) of Trilobites, 222
- Doflein, on eyes of deep-sea Crustacea, 148, 150
- Dohrn, 504, 513, 519
- Doleschall, 365
- Dolichopterus, 283, 291
- Doliomelus, 415
- Dolomedes fimbriatus, 416
- Dolops, 78
- Domed webs, 350
- Donachochara, 406
- Donnadieu, 457
- Dorippe, 185, 188
- Dorippidae, 188
- Doropygus, 66;
- D. pulex, 66
- Dorsal organ, of Phyllopoda, 22;
- of Cladocera, 39
- Doublure, 232
- Doyère, on Tardigrada, 481;
- on their systematic position, 483
- Doyeria, 485;
- Drassidae, 324, 396
- Drassinae, 396
- Drassus, 397;
- Drepanothrix, 53
- Dromia, 184;
- D. vulgaris, 184
- Dromiacea, 183;
- Dromidia, distribution, 200
- Dromiidae, 184
- Drymusa, 393
- Dufour, 385
- Dujardin, 464 n.;
- on systematic position of Tardigrada, 483
- Duplorbis, 95;
- D. calathurae, 99
- Dynomene, 184
- Dynomenidae, 184
- Dysdera, 394;
- Dysderidae, 317, 319, 336, 394
- Dysderina, 394
- Dysderinae, 394
- Ebalia, 188
- Echiniscoides, 485;
- Echiniscus, 480, 485;
- Echinoderms, Dendrogaster parasitic on, 94
- Echinognathus, 283
- Ecribellatae, 385
- Ectatosticta davidi, 393
- Ectinosoma, 62
- Edriophthalmata, 112, 121
- Eggs, of Phyllopoda, 32;
- of Cladocera, 44;
- of Copepoda, 59, 62, 66, 67, 71, 74;
- of Branchiura, 77;
- of Syncarida, 114;
- of Peracarida, 123;
- of Hoplocarida, 141;
- of Eucarida, 144;
- of Trilobites, 238;
- of Limulus, 275;
- of Pedipalpi, 309;
- of Spiders, 358;
- of Solifugae, 424;
- of Pseudoscorpions, 434;
- of Phalangidea, 442;
- of Acarina, 456;
- of Tardigrada, 478;
- of Pentastomida, 493;
- of Pycnogons, 520
- Ehrenberg, on systematic position of Tardigrada, 483
- Eleleis crinita, 396
- Ellipsocephalus, 224, 235, 247;
- E. hoffi, 248
- Embolobranchiata, 258, 259, 297 f.
- Emmerich, on facial suture of Trinucleus, 226
- Encephaloides, 193;
- Encrinuridae, 251
- Encrinurus, 227, 235, 251
- Endeis didactyla, 534;
- Endite, 9, 10
- Endopodite, 9, 10;
- of Trilobites, 237
- Endosternite, 257, 305, 330
- Endostoma, of Eurypterus, 287
- Engaeus, 157;
- E. fossor, distribution, 213
- Enoplectenus, 418
- Enterocola, 67;
- E. fulgens, 67
- Entomostraca, defined, 6;
- Entoniscidae, 130, 134
- Enyo, 400
- Enyoidae, 399
- 551Eoscorpius, 298
- Epeira, 409;
- E. angulata, 315, 409;
- E. basilica, 350, 351;
- web of, 351;
- E. bifurcata, 359;
- E. caudata, 359;
- E. cornuta, 409;
- E. cucurbitina, 372, 409;
- E. diademata, 335, 340, 343, 345, 359, 366, 380, 409;
- anatomy, 332;
- cocoon, 358;
- silk, 360;
- spinnerets, 325;
- E. labyrinthea, 350;
- E. madagascarensis, 360;
- E. mauritia, 349;
- E. pyramidata, 409;
- E. quadrata, 366, 409;
- E. triaranea, 350;
- E. umbratica, 409
- Epeiridae, 376, 377, 406
- Epeirinae, 408
- Ephippium, 48
- Epiblemum, 420
- Epicarida, 129;
- sex in, 105
- Epicaridian, larva of Epicarida, 130
- Epicoxite, of Eurypterus, 287
- Epidanus, 449
- Epigyne, 319, 333, 378
- Epipharynx, 459
- Epipodite, 9, 10
- Episininae, 402
- Episinus truncatus, 403
- Epistome, of Eurypterida, 291;
- Erber, 355, 356
- Eremobates, 429
- Eremobatinae, 429
- Eresidae, 398
- Eresus cinnaberinus, 398
- Eriauchenus, 411
- Erichthoidina, larva of Stomatopod, 143
- Ericthus, larva of Stomatopod, 143
- Erigone, 405
- Erigoninae, 404
- Eriophyes, 465;
- Eriophyidae, 464
- Eriphia, 191;
- E. spinifrons, 191
- Erlanger, von, on development and position of Tardigrada, 483
- Ero, 411;
- Eryonidae, 158;
- habitat, 204
- Eryonidea, 157
- Erythraeinae, 473
- Estheria, 21, 22, 23, 36;
- Eucarida, 114, 144 f.
- Euchaeta norwegica, 58
- Eucopepoda, 57 f.
- Eucopia australis, 119
- Eucopiidae, 113, 114, 118
- Eudendrium, Pycnogons on, 520
- Eudorella, 121
- Eukoenenia, 423;
- Eulimnadia, 36;
- Euloma, 230
- Eumalacostraca, 112 f.
- Eupagurinae, 180
- Eupagurus, 180;
- Euphausia pellucida, 145, 146
- Euphausiacea, 144
- Euphausiidae, 113, 114, 144;
- Eupodes, 471
- Euproöps, 278
- Eurycare, 232, 247
- Eurycercus, 53;
- Eurycide, 505, 533;
- Eurycididae, 533
- Eurydium, 485
- Euryopis, 404
- Eurypelma, 389;
- Euryplax, 195
- Eurypterida, 258, 278, 283 f.
- Eurypteridae, 290 f.
- Eurypterus, 283 f., 290, 291, 292;
- Eurytemora, 59;
- E. affinis, habitat, 206
- Eusarcus, 283, 291
- Euscorpiinae, 308
- Euscorpius, 298, 308;
- E. carpathicus, 299
- Eusimonia, 429
- Euterpe acutifrons, 61, 61;
- distribution, 203
- Euthycoelus, 389
- Evadne, 54;
- young, 47
- Excretory system (including Renal organs), in Crustacea, 12;
- Exner, on mosaic vision, 148
- Exopodite, 9, 10;
- of Trilobites, 237
- Eyes, compound, of Crustacea, 146, 147;
- physiology of, 148;
- of deep-sea Crustacea, 149;
- connexion with phosphorescent organs, 151;
- regeneration of, 6;
- of Trilobites, 227 f., 228;
- of Limulus, 271;
- of Eurypterida, 285;
- of Scorpions, 301;
- of Pedipalpi, 309;
- of Spiders, 315, 334; of Solifugae, 426;
- of Pseudoscorpions, 431;
- of Phalangidea, 442;
- of Acarina, 458;
- of Pycnogons, 517
- Fabre, on habits of Spiders, 298 f.;
- Facet, of Trilobites, 235
- Facial suture, 225 f., 232
- Falanga, 424
- False articulations, 444
- 552False-scorpions, 430
- Fecenia, 399
- Filistata, 391;
- Filistatidae, 319, 336, 391
- Finger-keel, 303
- Fixed cheek, 225, 226, 227
- Flabellifera, 124 f.
- Flabellum, 270
- Flacourt, 363
- Flagellum, in Solifugae, 426, 428;
- in Pseudoscorpions, 433
- Forbes, 374
- Ford, S. W., on development of Trilobites, 238
- Forel, on Lake of Geneva, 206
- Formicina, 405
- Formicinae, 405
- Formicinoides brasiliana, 318
- Fragilia, 535
- Free cheek, 225, 226, 227
- Fresh-water, Crustacea, 205 f.;
- Spiders, 357
- Furcilia (Metazoaea), larva of Euphausia, 145
- Fusulae, 325, 335
- Galathea, 169, 170;
- Galatheidae, 169
- Galatheidea, 169
- Galea, 433, 436
- Galena, 412
- Galeodes, 429, 527;
- Galeodidae, 428
- Gall-mites, 455, 464
- Gamasidae, 470
- Gamasinae, 470
- Gamasus, 460, 461, 463, 470;
- Gammaridae, 138
- Gammarus, 137, 138;
- Gampsonyx, 115, 118
- Garstang, on respiration of crabs, 186 n.
- Garypinae, 436, 437
- Garypus, 431, 436, 437, 438;
- Gaskell, 270, 277, 334
- Gasteracantha, 410;
- G. minax, 410
- Gasteracanthinae, 317, 409
- Gastrodelphys, 73
- Gastrolith, of Lobster, 155
- Gaubert, 525 n.
- Gebia littoralis, 167
- Gecarcinidae, 196
- Gecarcinus, 194, 195, 196
- Gegenbaur, 523
- Gelanor, 411, 412
- Gelasimus, 194, 196;
- Genal angle, 225
- Gené, 461
- Genital operculum, of Eurypterida, 288, 289, 291
- Genysa, 388
- Gerardia, Laura parasitic on, 93
- Geryon, 195
- Giardella callianassae, 73
- Gibocellidae, 448
- Gibocellum sudeticum, 447
- Giesbrecht, on Copepoda, 57;
- on phosphorescence, 59
- Gigantostraca, 258, 283 f.
- Gill-book, 270
- Glabella, 223
- Glabella-furrows, 223
- Glands, of Tardigrada, 481;
- Glaucothoe, larva of Eupagurus, 179, 180
- Gluvia, 429
- Glycyphagus, 466;
- Glyphocrangon, 164;
- Glyphocrangonidae, 164
- Glyptoscorpius, 283, 291, 294
- Gmelina, 138
- Gmogala scarabaeus, 394
- Gnamptorhynchus, 533
- Gnaphosa, 397
- Gnathia maxillaris, 124;
- life-history of 125
- Gnathiidae, 124
- Gnathobase, 10, 264
- Gnathophausia, 119, 256 n.;
- maxillipede of, 10
- Gnathostomata, 56
- Gnosippus, 429
- Goldsmith, 362
- Gonads, = reproductive organs, q.v.
- Gonodactylus, 143;
- G. chiragra, 143
- Gonoplacidae, 195
- Gonoplax, 195;
- G. rhomboides, 195
- Gonyleptidae, 442, 448, 449
- Goodsir, Harry, 535, 540
- Gordius, parasitic in Spiders, 368
- Gossamer, 342
- Graells, 364
- Graeophonus, 309
- Graff, von, on position of Tardigrada, 483
- Grapsidae, 193, 195;
- Graptoleberis, 53
- Grassi, 422
- Green gland, 110 (= antennary gland, q.v.)
- Gregarious Spiders, 340
- 553Grenacher, 517
- Griffithides, 251
- Gruvel, on Cirripedia, 80, 86
- Guérin-Méneville, 439
- Gurney, on Copepoda, 62;
- on Brachyuran metamorphosis, 181 n.
- Gyas, 450
- Gylippus, 429
- Gymnolepas, 89
- Gymnomera, 38, 54
- Gymnoplea, 57
- Hadrotarsidae, 394
- Hadrotarsus babirusa, 394
- Haeckel, on plankton, 203
- Haemaphysalis, 469
- Haematodocha, 322
- Haemocera, 64;
- Haemocoel, 5, 11
- Hahnia, 325, 416
- Hahniinae, 416
- Halacaridae, 472
- Halocypridae, 108
- Halosoma, 539
- Hannonia typica, 533
- Hansen, on Choniostomatidae, 76;
- Hansen and Sörensen, 422, 439, 443, 448
- Hapalogaster, 181;
- H. cavicauda, 178
- Hapalogasterinae, 181
- Harpactes hombergii, 395
- Harpacticidae, 61, 62;
- habitat, 206
- Harpedidae, 245
- Harpes, 225, 226, 230, 231, 234, 246;
- Harporhynchus, 53
- Harvest-bugs, 454, 473
- Harvestmen, 440, = Phalangidea, q.v.
- Harvest-spiders, 440, = Phalangidea, q.v.
- Harvesters, 440, = Phalangidea, q.v.
- Hasarius falcatus, 421
- Haustellata, 501 n.
- Haustoriidae, 137
- Haustorius arenarius, 137
- Hay, on name Lydella, 486 n.
- Heart, of Phyllopoda, 29;
- of Cladocera, 43;
- of Nebalia, 112;
- of Syncarida, 115;
- of Peracarida, 118;
- of Isopoda, 122;
- of Danalia, 132;
- of Amphipoda, 136;
- of Squilla, 142;
- of Eucarida, 144;
- of Limulus, 268;
- of Scorpions, 305;
- of Pedipalpi, 311;
- of Spiders, 331;
- of Solifugae, 427;
- of Pseudoscorpions, 434;
- of Phalangidea, 445;
- of Acarina, 460;
- of Pycnogons, 516
- Heart-water, 470
- Hedley, on home of cocoa-nut, 174
- Heligmonerus, 388
- Heller, 455
- Hemeteles fasciatus, 367;
- H. formosus, 367
- Hemiaspis, 278;
- H. limuloides, 278
- Hemioniscidae, 130
- Hemiscorpion lepturus, 307
- Hemiscorpioninae, 306, 307
- Henking, 447, 460
- Hentz, 367
- Herbst, on regeneration of eye, 6 n.
- Hermacha, 388
- Hermaphroditism, 15;
- Hermippus, 317, 399;
- H. loricatus, 400
- Hermit-crab, 167, 171;
- Hermit-lobster, 167
- Herrick, on the Lobster, 154
- Hersilia (Araneae), 401;
- H. caudata, 400
- Hersiliidae (Araneae), 326, 400
- Hersiliidae (Copepoda), 73
- Hersiliola, 401
- Heterarthrandria, 58
- Heterocarpus alphonsi (Pandalidae), phosphorescence, 151
- Heterochaeta papilligera, 60
- Heterocope, 59
- Heterogammarus, 138
- Heterometrus, 307
- Heterophrynus, 313
- Heteropoda venatoria, 414
- Heterostigmata, 471
- Heterotanais, 123
- Hexameridae, 91
- Hexathele, 390
- Hexisopodidae, 429
- Hexisopus, 429, 429
- Hexura, 391
- Hippa, 171;
- H. emerita, distribution, 202
- Hippidae, 171
- Hippidea, 170;
- habitat, 198
- Hippolyte, 164;
- Hippolytidae, 164;
- distribution, 199
- Hodge, George, 523, 540
- Hodgson, 508
- Hoek, on Cirripedia, 80;
- Holm, G., on Agnostus, 225;
- on Eurypterus, 285 n.
- Holmia, 236, 242, 247;
- Holochroal eye, 228
- Holopediidae, 51
- Holopedium, 38, 51
- Homalonotus, 222, 249;
- H. delphinocephalus, 223
- Homarus, 154;
- habitat, 200;
- excretory
- 554glands, 13;
- Homoeoscelis, 76
- Homola, 184;
- distribution, 205
- Homolidae, 184
- Homolodromia, 184;
- H. paradoxa, resemblance to Nephropsidae, 184
- Hood, of Phalangidea, 442, 452
- Hoplocarida, 114, 141
- Hoploderma, 468;
- H. magnum, 467
- Hoplophora, 468
- Horse-foot crab, = Limulus, q.v.
- Hoyle, on classification of Pentastomids, 495
- Hughmilleria, 283, 290, 292
- Humboldt, on Porocephalus, 488 n.
- Hutton, 424
- Huttonia, 398
- Hyale, 139
- Hyalella, 137, 139;
- Hyalomma, 469
- Hyas, 192, 193;
- distribution, 200
- Hyctia nivoyi, 421
- Hydrachnidae, 472
- Hydractinia, Pycnogons on, 523
- Hydrallmania, Pycnogons on, 524
- Hymenocaris, 112
- Hymenodora, 163
- Hymenosoma, 193;
- distribution, 200
- Hymenosomatidae, 193
- Hyperina, 140
- Hypochilidae, 393
- Hypochilus, 336, 393;
- H. thorelli, 393
- Hypoctonus, 312
- Hypoparia, 243
- Hypopus, 463
- Hypostome, of Trilobites, 233, 237;
- Hyptiotes, 349, 411;
- Iasus, 165, 167;
- distribution, 200
- Ibacus, 167
- Ibla, 88;
- Ichneumon flies, and Spiders, 367
- Icius, 421;
- I. mitratus, 382
- Idiops, 388
- Idothea, habitat, 211
- Idotheidae, 127
- Ihle, J. E. W., 526 n.
- Ilia, 188;
- Illaenus, 229, 231, 235, 249;
- I. dalmanni, 248
- Ilyocryptus, 40, 53
- Inachus, 192, 193;
- Integument, of Pycnogons, 518
- Irregular Spider-snares, 351
- Ischnocolus, 389
- Ischnothele dumicola, 390
- Ischnurinae, 306, 307
- Ischnurus ochropus, 307
- Ischnyothyreus, 394
- Ischyropsalidae, 451
- Ischyropsalis, 444, 451
- Isokerandria, 69 f.
- Isometrus europaeus, 306
- Isopoda, 121 f., 242
- Ixodes, 469;
- I. ricinus, 469
- Ixodidae, 469
- Ixodoidea, 455, 462, 468
- Janulus, 403
- Jaworowski, on vestigial antennae in a Spider, 263
- Johnston, George, 540
- Jumping-Spiders, 419
- Karshia, 429
- Karshiinae, 429
- Katipo, 363, 403
- King-crab, =Limulus, q.v.
- Kingsley, on Trilobites, 239, 243 n.;
- on breeding habits of Limulus, 271
- Kishinouye, on Limulus, 274, 275
- Klebs, on the frequency of human Pentastomids, 494
- Knight Errant, 540
- Koch, C., 397 n.
- Koch, L., 397 n.
- Kochlorine, 92;
- K. hamata, 93
- Koenenia, 422, 527, 528;
- K. mirabilis, 423
- Koltzoff, 15
- König, 524
- Koonunga cursor, 117;
- distribution, 211
- Koonungidae, 117
- Korschelt and Heider, on neuromeres in Arachnids, 263
- Kowalevsky, 513
- Kraepelin, 303, 306, 312 n., 428
- Kramer, 460
- Kröyer, 504, 526
- Labdacus, 418
- Labochirus, 312
- Labrum, of Trilobites, 233
- Labulla, 406
- Laches, 399
- Lachesis, 399
- Lacinia mobilis, 114
- Laemodipoda, 139
- Laenger, on the frequency of human Pentastomids, 494
- Lakes, characters of fauna of, 206;
- Lambrus, 192, 193;
- L. miersi, 193
- 555Lamproglena, 68
- Lampropidae, 121
- Lamprops, 121
- Langouste, 165
- Laniatores, 448
- Lankester, on Crustacean limb, 9;
- Laophonte littorale, 62;
- L. mohammed, 62
- Laseola, 404
- Lathonura, 53
- Latona, 51
- Latreille, 385, 408 n., 412, 504, 526
- Latreillia, 185;
- distribution, 205
- Latreillopsis, 185;
- L. petterdi, 185
- Latreutes ensiferus, habitat, 202
- Latrodectus, 362, 403;
- Laura, 93;
- L. gerardiae, 93
- Laurie, 309 n., 310, 311
- Leach, 526
- Lecythorhynchus armatus, 535
- Leeuwenhoek, on desiccation in Tardigrada, 484
- Leionymphon, 534
- Lendenfeld, von, 512, 523
- Lepas, 87;
- Lephthyphantes, 327, 406
- Lepidurus, 23, 24, 36;
- Leptestheria, 36;
- L. siliqua, 37
- Leptochela, 163
- Leptochelia, 122;
- L. dubia, dimorphism, 123
- Leptoctenus, 418
- Leptodora, 54;
- Leptodoridae, 54
- Leptoneta, 393
- Leptonetidae, 393
- Leptopelma, 389
- Leptoplastus, 247
- Leptostraca, 111, 242;
- Lernaea, 74;
- Lernaeascus, 73
- Lernaeidae, 74
- Lernaeodiscus, 95
- Lernaeopoda salmonea, 76
- Lernaeopodidae, 75
- Lernanthropus, 68;
- Lernentoma cornuta, 72
- Leuckart, on Pentastomida, 490, 492;
- Leuckartia flavicornis, 59
- Leucon, 121
- Leuconidae, 121
- Leucosia, 188
- Leucosiidae, 188;
- Leydigia, 53
- Lhwyd, Edward, on Trilobites, 221
- Lichadidae, 252
- Lichas, 222, 252
- Lichomolgidae, 70
- Lichomolgus, 71;
- Ligia oceanica, 128
- Ligidium, 129
- Lilljeborg, on Cladocera, 51 n.
- Limnadia, 21, 22, 36;
- Limnadiidae, 20, 23, 28, 29, 36
- Limnetis, 20, 21, 22, 36;
- Limnocharinae, 472
- Limnocharis aquaticus, 472
- Limulus, 256, 292;
- nervous system, 257;
- classification, 260, 276;
- segmentation, 260, 261, 262, 266, 270, 272;
- appendages, 263;
- habits, 265, 271;
- food, 267;
- digestive system, 268;
- circulatory system, 268;
- respiratory system, 269;
- excretory system, 270;
- nervous system, 270, 272;
- eggs and larvae, 274, 275;
- ecdysis, 274;
- used as food, 275–6;
- affinities, 277;
- fossil, 277;
- L. gigas, 276;
- L. hoeveni, 277;
- L. longispina, 264, 274;
- L. moluccanus, 264, 274, 276, 277;
- L. polyphemus, 261, 262, 264, 271;
- L. rotundicauda, 275, 277;
- L. tridentatus, 276
- Lindström, on facial suture of Agnostus and Olenellus, 225;
- Lingua, 459
- Linguatula, 488 n., 495;
- Linnaeus, 408 n., 502
- Linyphia, 406;
- Linyphiinae, 405
- Liobunum, 447, 450
- Liocraninae, 397
- Liocranum, 397
- Liphistiidae, 386
- Liphistioidae, 383
- Liphistius, 317, 383, 385, 386;
- L. desultor, 386
- Liriopsidae, 130
- Lispognathus thompsoni, eyes, 149
- 556Lister, M., 341, 342
- Lithodes, 181;
- Lithodidae, 181;
- evolution of, 176 f.
- Lithodinae, 181;
- Lithoglyptes, 92;
- L. varians, 93
- Lithotrya, 87;
- L. dorsalis, 87
- Lithyphantes, 404
- Littoral region, of sea, 197;
- of lakes, 206
- Liver (gastric glands), of Crustacea, 14;
- Lobster, distribution, 199;
- Lockwood, on habits of Limulus, 265, 271
- Loeb, 525 n.
- Loman, 331, 514, 525
- Lönnberg, 425
- Lophocarenum insanum, 405
- Lophogaster, 119
- Lophogastridae, 113, 114, 119
- Loricata, 165
- Lounsbury, 456, 461
- Love-dances, among spiders, 381
- Lovén, on Trilobites, 226
- Loxosceles, 393
- Lubbock, 375
- Lucas, 364
- Lucifer, 162
- Lung-books, 297, 308, 336;
- origin of, 305
- Lupa, 191;
- Lycosa, 417;
- Lycosidae, 359, 375, 381, 417
- Lydella, 479, 485;
- Lynceidae, 53;
- Lyncodaphniidae, 53
- Lyonnet, 319, 320
- Lyra, 328
- Lyriform organs, 325, 422
- Lysianassa, 137
- Lysianassidae, 137
- Lysianax punctatus, commensal with hermit-crab, 172
- M‘Cook, 334, 339, 340, 346, 350, 352 n., 365 n., 366, 367 n., 369 n.
- M‘Coy, F., on facial suture of Trinucleus, 226;
- on free cheek of Trilobites, 227
- M‘Leod, 336 n.
- Macrobiotus, 480, 485;
- M. ambiguus, 487;
- M. angusti, 486;
- M. annulatus, 486;
- M. coronifer, 487;
- M. crenulatus, 487;
- M. dispar, 487;
- M. dubius, 487;
- M. echinogenitus, 487;
- M. harmsworthi, 487;
- M. hastatus, 487;
- M. hufelandi, 480, 482, 483, 486;
- M. intermedius, 486;
- M. islandicus, 487;
- M. macronyx, 477, 483, 487;
- M. oberhäuseri, 486;
- M. orcadensis, 487;
- M. ornatus, 487;
- M. papillifer, 487;
- M. pullari, 487;
- M. sattleri, 487;
- M. schultzei, 480;
- M. tetradactylus, 478;
- M. tuberculatus, 487;
- M. zetlandicus, 486
- Macrocheira kämpferi, 192
- Macrohectopus (= Constantia), 138, 212
- Macrophthalmus, 196
- Macrothele, 390
- Macrothrix, 37, 53
- Macrura, 153 f.
- Macula, 233
- Maia, 193;
- Maiidae, 193
- Malacostraca, 110 f.;
- Malaquin, on Monstrilla, 63 n.
- Male Spider, devoured by female, 380
- Malmignatte, 364, 403
- Malpighian tubes or tubules, 12, 257, 311, 331, 427, 434, 460
- Mandibles, of Crustacea, 8;
- of Arachnida, 319
- Mange, 465
- Maracaudus, 449
- Margaropus, 469
- Marine Spiders, 415
- Marpissa, 421;
- Martins, Fr., 502
- Marx, 350
- Masteria, 390
- Mastigoproctus, 312
- Mastobunus, 449
- Matthew, G. F., on development of Trilobites, 238
- Matuta, 188;
- Maxilla, 8;
- Maxillary gland, 13
- Maxillipede, 8;
- Mecicobothrium, 391
- Mecostethi, 443, 447, 448
- Mecysmauchenius segmentatus, 411
- Meek, 363
- Megabunus, 450, 451
- Megacorminae, 308
- Megacormus granosus, 308
- Megalaspis, 222, 249
- Megalopa, compared to Glaucothoe, 180;
- of Corystes cassivelaunus, 183
- Mégnin, 455, 457
- Megninia, 466
- Meinert, 522 n.
- Meisenheimer, 511 n.
- 557Melanophora, 397
- Mena-vodi, 362
- Menge, 319, 368, 385
- Menneus, 410
- Mermerus, 449
- Merostomata, 258, 259 f.
- Mertens, Hugo, 524 n.
- Mesochra lilljeborgi, 62
- Mesonacis, 247;
- M. asaphoides, larva, 240
- Mesosoma, of Arachnida, 256;
- Mesothelae, 386
- Meta segmentata, 408
- Metamorphosis, of Cirripedia, 80;
- of Sacculina, 97;
- of Epicarida, 130, 133, 135;
- of Squilla, 142, 143;
- of Euphausia, 144;
- discovery of, in Decapoda, 153;
- of Lobster, 156;
- of Crayfish, 157;
- of Peneus, 159;
- primitive nature of, in Macrura, 161;
- of Loricata, 165, 166;
- of Hermit-crab, 179;
- of Brachyura, 181, 182;
- of Dromiacea, 182;
- of Trilobites, 239;
- of Limulus, 275;
- of Pseudoscorpions, 435;
- of Acarina, 462;
- of Pentastomida, 493 f.;
- of Pycnogons, 521 f.
- Metasoma, of Arachnida, 256;
- Metastigmata, 467
- Metastoma, of Trilobites, 234;
- Metazoaea, 182
- Metopobractus rayi, 405
- Metopoctea, 452
- Metridia, 59;
- M. lucens, distribution, 203
- Metronax, 398
- Metschnikoff, 435 n.
- Miagrammopes, 411
- Miagrammopinae, 411
- Micaria, 397;
- Micariinae, 397
- Micariosoma, 397
- Michael, 460, 461, 462, 466 n.
- Micrathena, 410
- Microdiscus, 225, 231, 245
- Microlyda, 486 n.
- Micrommata, 414;
- Microneta, 406
- Microniscidae, 130
- Migas, 387
- Miginae, 387
- Milne-Edwards, 504
- Milnesium, 480, 485;
- Miltia, 396
- Mimetidae, 411
- Mimetus, 411;
- M. interfector, 368
- Mimicry, in Spiders, 372
- Mimoscorpius, 312
- Miopsalis, 448
- Misumena, 412;
- Mites, = Acarina, q.v.
- Moggridge, 354, 355 n.
- Moggridgea, 387
- Moina, 37, 52;
- Mole-crab, 170
- Monochetus, 465
- Monolistra (Sphaeromidae), habitat, 211
- Monopsilus, 54
- Monostichous eyes, 301
- Monstrilla, 64
- Moustrillidae, 63
- Morgan, 517, 518, 521
- Mortimer, Cromwell, on Trilobites, 221
- Mosaic vision, 147
- Moseley, 523
- Moulting (Ecdysis), 154, 155, 225, 338
- Mouth, of Trilobites, 234
- Mud-mites, 472
- Müller, F., on Tanaids, 123
- Müller, O. F., on position of Tardigrada, 483
- Munidopsis, 170;
- Munnopsidae, 128
- Munnopsis typica, 127
- Murray, 455
- Murray, J., on British Tardigrada, 485
- Muscular system, in Tardigrada, 481;
- in Pentastomida, 490
- Mygale, 337, 386 n., 389
- Mygalidae, = Aviculariidae, q.v.
- Myrmarachne formicaria, 421
- Myrmecium, 397
- Myrtale perroti, 387
- Mysidacea, 118
- Mysidae, 113, 114, 119;
- Mysis, 120;
- Mysis-larva, of Lobster, 156;
- of Peneus, 161
- Mytilicola, 68
- Nanodamon, 313
- Nauplius, of Haemocera danae, 64;
- Nebalia, 111, 112, 114;
- Nebo, 307
- Neck-furrow, 224
- 558Nemastoma, 443, 451;
- Nemastomatidae, 451
- Nematocarcinus, 163
- Nemesia, 388;
- Neolimulus, 278, 279
- Neoniphargus, distribution, 216
- Neopallene, 537
- Nephila, 408;
- Nephilinae, 408
- Nephrops, 154;
- Nephropsidae, 154;
- resemblance to Dromiacea, 184
- Neptunus, 191;
- N. sayi, habitat, 202
- Nereicolidae, 73
- Nervous system, of Crustacea, 5;
- Neumann, 470
- Nicodaminae, 416
- Nicodamus, 416
- Nicothoe astaci, 68
- Nileus, 229, 249;
- N. armadillo, eye, 228
- Niobe, 249
- Niphargoides, 138
- Niphargus, 137, 138;
- Nogagus, 73
- Nops, 315, 336, 395
- Norman, A. M., 540
- Notaspis, 467
- Nothrus, 468
- Notodelphys, 66
- Notostigmata, 473
- Nyctalops, 312
- Nycteribia (Diptera), 526
- Nymph, 463
- Nymphon, 503, 536;
- N. brevicaudatum, 507, 536;
- N. brevicollum, 511, 521;
- N. brevirostre, 503, 504, 506, 508, 509, 541, 542;
- N. elegans, 506, 542;
- N. femoratum, 541;
- N. gallicum, 541;
- N. gracile, 511, 541, 542;
- N. gracilipes, 542;
- N. grossipes, 541;
- N. hamatum, 512;
- N. hirtipes, 542;
- N. horridum, 537;
- N. johnstoni, 541;
- N. leptocheles, 542;
- N. longitarse, 541, 542;
- N. macronyx, 542;
- N. macrum, 542;
- N. minutum, 541;
- N. mixtum, 541;
- N. pellucidum, 541;
- N. rubrum, 541, 542;
- N. serratum, 542;
- N. simile, 541;
- N. sluiteri, 542;
- N. spinosum, 541;
- N. stenocheir, 542;
- N. strömii, 509, 541
- Nymphonidae, 536
- Nymphopsinae, 535 n.
- Nymphopsis, 534, 535 n.;
- Obisiinae, 436, 437
- Obisium, 436, 438
- Ochyrocera, 393
- Octomeridae, 91
- Octomeris, 91
- Ocyale mirabilis, 416
- Ocypoda, 194, 196;
- Ocypodidae, 196
- Oecobiidae, 386 n., 392
- Oecobius, 392;
- Oe. maculatus, 392
- Oehlert, on facial suture of Trinucleus, 226
- Ogovia, 448
- Ogygia, 249
- Oiceobathes, 535
- Oithona, 61;
- Olenelloides, 247;
- O. armatus, 247
- Olenellus, 225, 227, 232, 236, 247
- Olenidae, 247
- Olenus, 232, 247;
- O. truncatus, 248
- Oligolophus, 450;
- Olpium, 436, 437;
- O. pallipes, 437
- Ommatoids, 310, 311, 312
- Oncaea, 69;
- O. conifera, phosphorescence, 60
- Oncaeidae, 69
- Oniscoida, 128
- Oniscus, 129
- Ononis hispanica, Spiders on, 419
- Onychium, 324
- Oomerus stigmatophorus, 539
- Oonopidae, 336, 393
- Oonops, 394;
- Oorhynchus, 507, 535;
- O. aucklandiae, 535
- Oostegites, of Malacostraca, 114
- Operculata, 89, 91
- Ophiocamptus (Moraria), 62;
- O. brevipes, 62
- Opilioacarus, 454, 473;
- Opiliones (= Phalangidea, q.v.), 440
- Opisthacanthus, 307
- Opisthoparia, 244
- Opisthophthalmus, 307
- Opisthothelae, 386
- Opopaea, 394
- Orchestia, 139;
- Orchestina, 394
- Oribata, 467
- Oribatidae, 457, 458, 459, 460, 462, 467;
- anatomy, 459
- 559Orithyia coccinea, 524, 540
- Ornithodoros, 469;
- Ornithoscatoides, 374
- Orometopus, 226, 245;
- O. elatifrons, 230
- Ortmann, on Brachyura, 181 n.;
- Ostracoda, 107;
- pelagic, 202
- Oudemans, 528 n.
- Ovary, of Cladocera, 44, 45;
- Oxynaspis, 88
- Oxyopes, 419;
- O. lineata, 419
- Oxyopidae, 419
- Oxyptila, 412
- Oxyrhyncha, 191 f.;
- Oxystomata, 185 f.;
- Pachycheles, 170;
- P. panamensis, distribution, 202
- Pachygnatha, 407;
- Pachygrapsus, 196;
- Pachylasma giganteum, 91
- Pachylomerus, 388
- Pachysoma, 69
- Pagurian, 180;
- Paguridea, 171
- Pagurinae, 180
- Palaemon, 164;
- Palaemonetes, 164;
- Palaemonidae, 159, 164
- Palaeocaris, 115, 118
- Palaeophonus, 294, 298
- Palamnaeus, 307;
- P. swammerdami, tarsus, 304
- Palinuridae, 167
- Palinurus, 165, 167;
- Pallene, 505, 537;
- Pallenidae, 537
- Pallenopsis, 506, 511;
- Palp, of Pycnogons, 507
- Palpal organ, 322, 378
- Palpebral lobe, 227
- Palpigradi, 258, 422
- Palpimanidae, 323, 325, 398
- Palpimanus, 398
- Panamomops diceros, 405
- Pandalidae, 164
- Pandalus, 164;
- P. annulicornis, 164
- Pandinus, 307
- Panoplax, 195
- Pantopoda, 501 n. (= Pycnogonida, q.v.)
- Panulirus, 165, 167
- Parabolina, 232, 247
- Parabolinella, 247
- Parabuthus, 298;
- Paradoxides, 222, 232, 236, 247;
- P. bohemicus, 246
- Paragaleodes, 429
- Paralomis, 179, 181
- Paranaspides, 117;
- Paranebalia, 242
- Paranephrops, 157;
- distribution, 213
- Paranthura, 124
- Parantipathes, Synagoga parasitic on, 94
- Paranymphon, 507;
- P. spinosum, 542
- Parapagurus, 180
- Parapallene, 537
- Parapeneus, 162;
- P. rectacutus, 159
- Parapylocheles scorpio, eyes, 149
- Parasiro, 448;
- P. corsicus, 448
- Parasites, in Tardigrada, 484
- Parasitic castration, 100, 136
- Parastacidae, 157;
- distribution, 213
- Parastacus, 157;
- distribution, 213
- Paratropidinae, 387
- Paratropis scrupea, 387
- Parazetes auchenicus, 533
- Pardosa, 417;
- Pariboea spinipalpis, 534
- Parthenogenesis, in Phyllopoda, 32;
- Parthenope, 193;
- P. investigatoris, 192
- Parthenopidae, 193
- Pasiphaea, 163
- Pasiphaeidae, 163
- Pasithoe, 532;
- Pasithoidae, 532
- Patten, 270, 271, 277
- Patten and Redenbaugh, on Limulus, 266, 270, 272
- Paturon, 319, 320
- Peckham, 376, 377, 378, 381, 382
- Pecten, 328
- Pectines, of Scorpions, 302, 302;
- Pedicle, 317
- Pedipalpi, 258, 308;
- 560Pedipalpi (appendages), 263, 303, 309, 321, 422, 426, 433, 440, 458
- Pedunculata, 84
- Pelagic Crustacea, marine, 202;
- lacustrine, 207
- Pelops, 467
- Peltiidae, 63
- Peltogaster, 95;
- Peltura, 247
- Peneidae, 162
- Peneidea, 158, 162;
- metamorphosis, 159
- Penella sagitta, 74
- Peneus, 158, 162;
- Pentanymphon, 504, 537
- Pentaspidae, 87
- Pentastoma, 488 n.;
- Pentastomida, 258, 488 f.;
- Pephredo hirsuta, 535, 541
- Peracantha, 43, 53;
- alimentary canal, 43
- Peracarida, 114, 118
- Pereiopod, defined, 110;
- Periegops hirsutus, 393
- Peroderma cylindricum, 75
- Petrarca bathyactidis, 93
- Pettalus, 448
- Pezomachus gracilis, parasitic in cocoons of Spiders, 367
- Phacopidae, 249
- Phacopini, 243
- Phacops, 223, 232, 235, 249;
- Phaeocedus braccatus, 397
- Phagocytes, in Danalia, 132
- Phalangidea, 258, 440 f.;
- Phalangiidae, 449
- Phalangiinae, 450
- Phalangium, 444, 450, 526;
- Phalangodes, 449;
- Phalangodidae, 448
- Phanodemus, 535
- Phidippus, 421;
- Philichthyidae, 73
- Philichthys, 73;
- P. xiphiae, 73 n.
- Phillipsia, 251;
- P. gemmulifera, 250
- Philodrominae, 413
- Philodromus, 413;
- Philoscia muscorum, 129
- Pholcidae, 336, 401
- Pholcus, 320, 401;
- P. phalangioides, 401
- Phoroncidia, 404;
- P. 7–aculeata, 318
- Phoroncidiinae, 317, 404
- Phosphorescence, of Copepoda, 59;
- Phosphorescent organs, of Euphausiidae, 145;
- of Stylocheiron mastigophorum, 151
- Phoxichilidae, 539
- Phoxichilidiidae, 538
- Phoxichilidium, 506, 512, 520, 521 n., 523, 525, 538;
- Phoxichilus, 505, 512, 539;
- Phreatoicidae, 136;
- Phreatoicidea, 136
- Phreatoicopsis, 136;
- distribution, 211
- Phreatoicus, 136;
- Phronima, 140;
- P. sedentaria, 140
- Phrynarachne, 414;
- Phrynichinae, 313
- Phrynichus, 313
- Phrynidae, 309, 310, 312
- Phrynopsis, 313
- Phrynus, 312
- Phryxidae, 130
- Phyllocarida, 111, 242
- Phyllocoptes, 465
- Phyllopoda, 19 f.;
- Phyllosoma, larva of Palinurus, 166
- Phytoptidae, 464
- Phytoptus, 464 n., 495 (= Eriophyes, q.v.)
- Pickard-Cambridge, F., 352
- Pickard-Cambridge, O., 318, 321 n., 323 n., 359 n., 372, 374, 380, 385, 401 n., 436, 438, 450, 451, 452
- Pillai, 375
- Pilumnus, 191
- Pinnotheres pisum, 195
- Pinnotheridae, 195
- 561Pipetta, 514, 533;
- P. weberi, 533
- Pirata, 417
- Piriform glands, 335, 349
- Pisa, 193
- Pisaura mirabilis, 416
- Pisauridae, 416
- Placoparia, 251
- Plagiostethi, 443, 447, 449, 452
- Plagula, 317
- Planes minutus, habitat, 202
- Plankton, characters of, 203;
- Plastron, 316
- Plate, on Tardigrada, 481, 482, 484
- Plator insolens, 415
- Platoridae, 415
- Platyarthrus hoffmannseggii, 129
- Platyaspis, 121
- Platybunus, 450, 451
- Platycheles, 535
- Plectreurys, 393
- Pleopod, defined, 110
- Pleura, 234 f.
- Pleurocrypta microbranchiata, 133
- Pleuromma, 59;
- Pliobothrus symmetricus, Pycnogon larvae in, 523
- Pocock, 298, 308 n., 312, 328, 329, 425 n., 534 n.
- Podasconidae, 130
- Podogona, 258, 439
- Podon, 54
- Podophthalmata, 112
- Podoplea, 61
- Podosomata, 501 n. (= Pycnogonida, q.v.)
- Poecilotheria, 390
- Poisonous hairs, of Spiders, 365
- Pollicipes, 84;
- Pollock, 340
- Poltyinae, 410
- Poltys, 410;
- P. ideae, 318
- Polyartemia, 36;
- Polyaspidae, 84
- Polycopidae, 109
- Polygonopus, 539
- Polyphemidae, 54;
- Polyphemus, 47, 54;
- Polysphincta carbonaria, parasitic on Spiders, 368
- Pompeckj, on Calymenidae, 244
- Pompilus, 368
- Pontellidae, 60
- Pontoporeia, 137;
- Porcellana, 168, 170;
- Porcellanidae, 170;
- habitat, 198
- Porcellio, 129
- Porcupine, 540
- Porhomma, 406
- Porocephalus, 488 n., 495;
- P. annulatus, 490, 496;
- P. aonycis, 496;
- P. armillatus, 496;
- P. bifurcatus, 496;
- P. clavatus, 496;
- P. crocidura, 496;
- P. crotali, 496;
- P. geckonis, 496;
- P. gracilis, 496;
- P. heterodontis, 496;
- P. indicus, 496;
- P. lari, 496;
- P. megacephalus, 497;
- P. megastomus, 497;
- P. moniliformis, 497;
- P. najae sputatricis, 497;
- P. oxycephalus, 497;
- P. platycephalus, 497;
- P. proboscideus, 493, 494;
- P. protelis, larva, 495;
- P. subuliferus, 497;
- P. teretiusculus, 489, 491, 492, 492, 497;
- P. tortus, 497
- Portunidae, 191
- Portunion, 134;
- Portunus, 191
- Potamobius (= Astacus), 157;
- distribution, 213
- Potamocarcinus, 191;
- distribution, 213
- Potamon, 191
- Potamonidae, 191
- Praniza, larva of Gnathia, 125
- Prawn, 151, 153, 158, 164, 198;
- Pre-epistome, 443
- Prestwichia (Euproöps), 275, 278, 279
- Preyer, on anabiosis in Tardigrades, 484
- Prionurus, 298, 299
- Prismatic eye, of Trilobites, 229
- Procurved eyes, 316
- Prodidomidae, 395
- Prodidomus, 396
- Proëtidae, 251
- Proëtus, 251;
- P. bohemicus, 248
- Prokoenenia, 423;
- Prolimulus, 279
- Promesosternite, in Limulus, 264
- Proparia, 244
- Prosalpia, 450
- Prosoma, of Arachnida, 260;
- Prosthesima, 397
- Prostigmata, 471
- Protaspis, 239, 239, 240
- Proteolepas, 94;
- P. bivincta, 94
- Protocaris, 243
- Protolenus, 247
- Protolimulus, 279
- Protolycosa anthrocophila, 383
- Przibram, on regeneration in Crustacea, 156
- Psalidopodidae, 164;
- habitat, 204
- Psalidopus, 164
- 562Psalistops, 389
- Psechridae, 399
- Psechrus, 399
- Pseudalibrotus, 137
- Pseudidiops, 388
- Pseudocuma, 121;
- distribution, 215
- Pseudocumidae, 121
- Pseudoniscus, 279
- Pseudopallene, 511, 537;
- Pseudoscorpiones, 258, 430 f.;
- Pseudo-stigmatic organs, 467
- Pseudozoaea, larva of Stomatopod, 143
- Pterocuma, 121
- Pterolichus, 466
- Pteronyssus, 466
- Pterygometopus, 249
- Pterygotus, 283, 291, 292;
- P. osiliensis, 290
- Ptychoparia, 247
- Pucetia viridis, 419
- Pupa, of Cirripedia, 81, 82
- Purcellia, 448
- Pychnogonides, 501 n.
- Pycnogonida, 501 f.;
- body, 505;
- chelophores, 505;
- palpi, 507;
- ovigerous legs, 507;
- glands, 511;
- alimentary system, 513;
- circulatory system, 516;
- nervous system, 516;
- eyes, 517;
- integument, 518;
- reproductive organs, 519;
- eggs, 520;
- development, 520;
- habits, 524;
- systematic position, 525;
- classification, 528 f.;
- British species, 540 f.
- Pycnogonidae, 539
- Pycnogonum, 503, 539;
- Pygidium, 235
- Pylocheles, 180;
- P. miersii, 173
- Pylochelidae, 180;
- habitat, 204
- Pylopagurus, 180;
- Pyrgoma, 92
- Rachias, 388
- Railliet, on classification of Pentastomids, 495
- Ranina dentata, 188
- Raninidae, 188
- Rastellus, 320, 387
- Ratania, 68;
- mouth, 63
- Réaumur, 360
- Recurved eyes, 316
- Red spider, 455, 472
- Red-water, 456
- Regeneration, of Crustacean limbs, 155, 156
- Regillus, 414
- Reichenbach, on embryology of Astacus, 12
- Reighardia, 495, 497;
- hosts of, 497
- Remipes, 171;
- R. scutellatus, 171
- Remopleurides, 232, 247;
- Reproduction (incl. Breeding), of Cladocera, 43 f.;
- Reproductive (generative) organs, of Crustacea, 15;
- Respiration, of Crustacea, 16;
- Respiratory organs, of Arachnids, 256;
- Rhagodes, 425, 429
- Rhagodinae, 429
- Rhax, 429
- Rhipicentor, 469
- Rhipicephalus, 469;
- R. sanguineus, 470
- Rhizocephala, 95 f.;
- Rhomphaea, 402
- Rhopalorhynchus, 532;
- Rhynchothoracidae, 535
- Rhynchothorax, 505, 535;
- Ricinulei, 439
- Robber-crab, 173
- Roncus, 436, 438
- Rucker, 423
- Rudolphi, on Pentastoma, 488 n.
- Sabacon, 451
- Sabelliphilus, 71
- Sacculina, 95;
- Saitis, 421;
- Salter, on facial suture of Trinucleus, 226;
- on classification of Trilobites, 243
- Salticidae, 419
- Salticus, 420;
- Sao, 235, 247;
- S. hirsuta, development, 239
- Sapphirina, 69;
- Sarcoptes, 466;
- S. mutans, 466
- Sarcoptidae, 455, 466
- Sarcoptinae, 466
- Sars, G. O., on Calanidae, 58;
- Savigny, 526
- Scaeorhynchus, 533
- Scalidognathus, 388
- Scalpellum, 84, 85;
- Scaphognathite, 152
- Scapholeberis, 39, 52;
- S. mucronata, 52
- Schimkewitsch, 527, 534
- Schizochroal eye, 228, 229
- Schizonotidae, 310, 312
- Schizonotus, 312
- Schizopoda, 112;
- Schizorhynchus, 121
- Schmeil, on fresh-water Copepoda, 59, 62
- Schultze, on position of Tardigrada, 483
- Scipiolus, 535
- Sclerocrangon, distribution, 200
- Sclerosoma, 450;
- S. quadridentatum, 450
- Sclerosomatinae, 449
- Scodra, 390
- Scopula, 324, 324, 389 n.
- Scorpio, 305, 307;
- Scorpion, 297 f.
- Scorpionidae, 306
- Scorpionidea, 258, 297 f.;
- habits, 298;
- senses, 299;
- poison, 299, 301;
- mating habits, 300;
- external structure, 301;
- prosoma, 301;
- pre-cheliceral segment, 301;
- development of eyes, 301;
- mesosoma, 302;
- metasoma, 303;
- appendages, 303;
- pedal spurs, 304, 306, 307, 308;
- tibial spurs, 304, 306, 307, 308;
- internal anatomy, 304;
- alimentary canal, 304;
- vascular system, 305;
- nervous system, 305;
- endosternite, 305;
- generative organ, 305;
- development of, 305;
- classification, 306;
- fossil, 298;
- resemblance to Eurypterids, 292
- Scorpioninae, 306, 307
- Scorpiops, 308
- Scotinoecus, 390
- Scott, on fish-parasites, 69 n.
- Scourfield, on Cladocera, 51 n.
- Scutum, of Spiders, 317, 394;
- of Ticks, 469
- Scyllarus, 167;
- Scytodes thoracica, 393
- Scytodidae, 393
- Segestria, 395;
- Segestriinae, 395
- Segmentation, of Crustacea, 5 f.;
- Selenopinae, 414
- Selenops, 414
- Semper, 521 n.
- Senoculidae, 418
- Senoculus, 418
- Sense-organs, of Arachnids, 257;
- Sergestes, 162
- Sergestidae, 162;
- Serolidae, 126
- Serolis, 126;
- Serrula, 322, 433
- Sesarma, 196;
- distribution, 213
- Setella, 61
- Sex, in Crustacea, 100;
- in Trilobites, 235
- Sexual dimorphism, of Copepoda, 57, 67, 75;
- Sheet-webs, 352
- Shell-gland, 13
- Shipley, A. E., introduction to Arachnida, 253 f.;
- Shrimp, 153, 158, 164, 198, 199
- Shumardia, 245
- Shumardiidae, 245
- Sicariidae, 327, 393
- Sicarius, 393
- Sida, 51;
- Sididae, 51;
- Siebold, von, 464 n.
- Sigilla, 410
- Silvestri, 473 n.
- Simocephalus, 52;
- Simon, 303, 314 n., 326, 385, 386 n., 387, 391 n., 397 n., 400, 401 n., 406, 408 n., 414 n., 418, 431, 433, 449, 452
- 564Singa, 409
- Sintula, 406
- Siphonostomata, 56
- Siriella, 120
- Siro, 448
- Sironidae, 448
- Sitalces, 449
- Slimonia, 283, 290, 292;
- S. acuminata, 291
- Smith, F., 367
- Smith, G. on Crustacea, 1 f.
- Smith, H., 373
- Smith and Kilborne, 456
- Snouted Mites, 458, 471
- Solenopleura, 247
- Solenysa, 405
- Solifugae, 258, 423 f.;
- Solpuga, 429;
- S. sericea, 425
- Solpugae, 423
- Solpugidae, 429
- Solpuginae, 429
- Spallanzani, on desiccation of Tardigrada, 484
- Sparassinae, 323, 414
- Sparassus, 414
- Spencer, on Pentastomida, 489 n., 490
- Spermatheca, 15
- Spermatophore, 15
- Spermatozoa, of Crustacea, 15;
- of Malacostraca, 114
- Spermophora, 401
- Sphaerexochus, 251
- Sphaeroma, habitat, 211
- Sphaeromidae, 126
- Sphaeronella, 76
- Sphaerophthalmus, 232, 247;
- S. alatus, eye, 228
- Spiders, 314 f.;
- external structure, 314, 316, 317;
- appendages, 319 f.;
- rostrum, 320;
- maxilla, 321;
- palpal organs, 321;
- tarsi, 324;
- spinnerets, 325;
- stridulating organs, 327, 404;
- internal anatomy, 329, 330;
- alimentary system, 329;
- vascular system, 331;
- generative system, 333;
- nervous system, 333;
- sense-organs, 333;
- eyes, 315, 334, 375;
- spinning glands, 335;
- respiratory organs, 318, 336;
- coxal glands, 337;
- poison-glands, 337;
- ecdysis, 338;
- early life, 338;
- ballooning habit, 341, 342;
- webs, 343 f.;
- nests, 354;
- cocoons, 358, 358;
- commercial use of silk, 359;
- poison, 360;
- fertility, 365;
- cannibalism, 367;
- enemies, 368;
- protective coloration, 371;
- senses, 375 f.;
- sight, 375;
- hearing, 376;
- touch, 334;
- intelligence, 377;
- mating habits, 378;
- fossil, 383;
- classification, 384 f.
- Spinning glands, 335
- Spinning Mites, 472
- Spiroctenus, 388
- Spongicola, 162
- Squilla, 141, 141, 142, 143;
- Squillidae, 114, 143;
- compared with Loricata, 166
- Stalita, 395
- Stasinopus caffrus, 387
- Staurocephalus, 251
- Steatoda, 404;
- Stebbing, on Amphipods, 137;
- Stecker, 447
- Stegosoma testudo, 318
- Stenochilus, 398
- Stenochotheres, 76;
- Stenocuma, 121
- Stenopodidae, 162
- Stenopus, 162
- Stenorhynchus, 192, 193
- Stephanopsinae, 414
- Stephanopsis, 414
- Stiles, on larval Pentastomids, 493, 494
- Stomatopoda, 114, 141 f.
- Storena, 399
- Strabops, 283;
- eyes, 290
- Strauss-Durckheim, on Limulus, 277
- Streblocerus, 53
- Streptocephalus, 25, 35;
- Stridulating organs, in Arachnids, 257, 327, 327, 404
- Stygina, 249
- Style, of palpal organ of Spiders, 322
- Stylocellus, 448
- Stylonurus, 283, 291;
- S. lacoanus, 293
- Sunaristes paguri, 63
- Sun-spiders, 423
- Sybota, 410
- Sylon, 95;
- sex, 99
- Symphysurus, 249
- Synageles, 421;
- Synagoga mira, 94
- Syncarida, 114
- Synemosyna, 420, 421;
- S. formica, 373
- Synhomalonotus, 249
- Syringophilus, 455, 473
- Tachidius brevicornis, 62;
- T. littoralis, 62
- Tachypleinae, 276
- Tachypleus, 276;
- Talitridae, 139
- Talitrus, 139;
- Talorchestia, 139
- Tanaidae, 122
- Tanais, 122
- Tanganyika, Lake, prawns of, 212
- 565Tanystylum, 505, 535;
- Taracus, 451
- Tarantella, 361
- Tarantism, 361
- Tarantula (Spider), 361
- Tarantula, 313;
- T. reniformis, 312
- Tarantulidae, 310, 312
- Tarantulinae, 313
- Tardigrada, 258, 477 f.;
- Tarentula, 417
- Tarsonemidae, 471
- Tartaridae, 312, 527
- Tealia, Pycnogonum on, 524
- Tegenaria, 416;
- Telema tenella, 393
- Telson, 6, 7;
- of Phyllopoda, 22
- Temora longicornis, distribution, 203
- Tethys (Mollusca), Pycnogon larva on, 524
- Tetrabalius, 312
- Tetrablemma, 315, 404;
- T. medioculatum, 318
- Tetraclita, 91, 92
- Tetragnatha, 407;
- T. extensa, 372
- Tetragnathinae, 407
- Tetrameridae, 92
- Tetranychinae, 472
- Tetranychus, 472;
- Tetraspidae, 88
- Teutana, 404
- Teuthraustes, 308
- Texas fever, 456, 470
- Thalassinidea, 167
- Thamnocephalus, 36;
- Thanatus, 414;
- Thaumasia, 416
- Thelphusa, 191;
- Thelphusidae, 191
- Thelyphonellus, 312
- Thelyphonidae, 309, 312
- Thelyphonus, 309, 310, 312;
- resemblance to Eurypterids, 294
- Theotina, 393
- Theraphosa, 389;
- Theraphosae, 319
- Theraphosidae, 391 n.
- Theridiidae, 327, 351, 401
- Theridion, 376, 403;
- Theridioninae, 403
- Theridiosoma argenteolum, 407
- Theridiosomatinae, 407
- Thersites gasterostei, 71
- Thomisidae, 323, 324, 369, 371, 381, 412
- Thomisinae, 412
- Thomisus, 412;
- T. onustus, 413
- Thompson, D’A. W., on Pycnogonida, 499 f.
- Thoracica, 84
- Thorax, of Trilobites, 234
- Thorell, 383
- Thyas petrophilus, 460
- Tibellus, 414;
- Tick fever, 469
- Ticks, 468 f.;
- Titanodamon, 313
- Tityus, 298, 306
- Tmeticus, 406
- Tomoxena, 403
- Torania, 414
- Tracheae, in Arachnida, 256;
- Trap-door Spiders, 354, 387, 388
- Trechona venosa, 390
- Triarthrus, 230, 234, 236, 247;
- Trichoniscus, 129
- Trigonoplax, 193
- Trilobita, 219 f.
- Trilobite larva, of Limulus, 275, 276
- Trimerocephalus, 249;
- T. volborthi, 229
- Trinucleidae, 230, 245
- Trinucleus, 225, 226, 230, 231, 236, 238, 245;
- Tripeltis, 312
- Trithena tricuspidata, 404
- Trithyreus, 312
- Triton, cruise of the, 540
- Trochantin, 433, 436, 449, 451, 452
- Trochosa, 417;
- Troglocaris, 163;
- T. schmidtii, habitat, 210
- Trogulidae, 439, 442, 444, 452
- Trogulus, 452;
- Trombidiidae, 472
- Trombidiinae, 473
- Trombidium, 473;
- Tropical zone (marine), 201
- Trouessart, 455
- Trygaeus, 506, 535;
- T. communis, 535
- Tubicinella trachealis, 91
- Tubularia, Pycnogons on, 522, 525
- Tubuliform glands, 335, 349
- Tulk, 445, 446, 461 n.
- Turret-spider, 357
- Turrilepas, 84;
- T. wrightianus, 84
- 566Tylaspis, 179
- Typhlocarcinus, 195
- Typopeltis, 312
- Tyroglyphidae, 466 n.
- Tyroglyphinae, 466
- Tyroglyphus, 464, 466, 481;
- Uliodon, 418
- Uloboridae, 350, 410
- Uloborinae, 410
- Uloborus, 352, 410;
- Unguis, 319, 320
- Uroctea, 392;
- U. durandi, 392
- Urocteidae, 386, 392
- Urodacinae, 306, 307
- Urodacus, 307
- Uroplectes, 306
- Uropoda, 471
- Uropodinae, 471
- Uroproctus, 312
- Uropygi, 312
- Usofila, 393
- Valvifera, 127
- Vancoho, 362
- Vectius, 415
- Vejdovský, 435 n.
- Vejovidae, 306, 308
- Vejovis, 308
- Vermiformia, 464
- Verruca, 89, 91
- Verrucidae, 91
- Vesicle, of Scorpion, 303
- Vinson, 349, 360, 362
- Virbius, 164;
- Virchow, on human Pentastomids, 494
- Viscid globules, on Spider web, 347
- Waite, 13
- Walckenaer, 365, 386 n., 408 n.
- Walckenaera, 405;
- W. acuminata, 405
- Walcott, on appendages of Trilobites, 236;
- Wallace, 381
- Wall-spider, 369
- Warburton, C., on Arachnida, 295 f., 344 n., 349 n., 378 n.
- Ward, on Reighardia, 495
- Wasps and Spiders, 368
- Water-mites, 460, 471, 472
- Water-spider, 357, 415
- Weismann, on Cladocera, 44, 49
- Weldon, W. F. R., on excretory glands, 13;
- Westring, 327, 384
- Whale-louse, 502
- Whip scorpions, 309
- White, Gilbert, 342
- Wilder, 366
- Willemoesia, 157;
- W. inornata, 158
- Winkler, 463
- With, 473 n.
- Wolf-spiders, 341, 356, 359, 369, 375, 377, 381, 417
- Wood-Mason, 328
- Woods, H., on Trilobita, 219 f.;
- Xanthidae, 191
- Xantho, 191;
- habitat, 198
- Xenobalanus globicipitis, 92
- Xiphocaris, 163;
- distribution, 210
- Xiphosura, 258, 259 f.;
- Xiphosura, 276;
- X. polyphemus, 276
- Xiphosuridae, 276
- Xiphosurinae, 276
- Xysticus, 412;
- Zacanthoides, 247
- Zaeslin, on the frequency of human Pentastomids, 494
- Zeriana, 429
- Zilla, 409;
- Zimris, 396
- Zoaea, compared with Cumacea, 120;
- Zodariidae, 317, 399
- Zodarion, 399
- Zora, 397;
- Z. spinimana, 396
- Zoropsis, 415
- Zoropsidae, 415
1. The muscles are to a certain extent segmented in correspondence with the limbs; and the heart, in Phyllopoda and Stomatopoda, may have segmentally arranged ostia.
2. Herbst, Arch. Entwick. Mech. ii., 1905, p. 544.
3. Quart. J. Micr. Sci. xlix., 1906, p. 469.
4. Present in Nebalia.
5. As many as 37 ambulatory appendages may be present.
6. Quart. J. Micr. Sci. xxi., 1881, p. 343.
7. Abhandl. Senckenberg. Nat. Gesellsch. xiv., 1886.
8. The Cumacea, Anaspidacea, and certain Isopods possess a maxillary gland only.
9. Quart. J. Micr. Sci. xxxii., 1891, p. 279.
10. Arch. Zool. Exp. (2) x., 1892, p. 57.
11. Bull. Mus. Comp. Zool. Harvard, xxxv., 1899, p. 152.
12. Arch. f. mikr. Anat. lxvii., 1906, p. 364.
13. Vol. x., 1897, pp. 97, 264.
14. For this use of the term Branchiopoda, cf. Boas, Morph. Jahrb. viii., 1883, p. 519.
15. Bernard, “The Apodidae,” Nature Series, 1892.
16. Arb. Zool. Inst. Wien, vi., 1886, p. 267.
17. I do not understand Packard’s account of the telson in Thamnocephalus.
18. The nomenclature here adopted is not that of Lankester.
19. [The red pigment in Lernanthropus, see p. 68, has been shown to be not haemoglobin, so that the presence of this substance in Phyllopod blood becomes doubtful.—G.S.]
20. Zeitschr. wiss. Zool. lxxi., 1902, p. 508.
21. Cf. Gaskell, Journ. Anat. Physiol. x., 1876, p. 153.
22. Bernard’s statement that Apus is hermaphrodite seems based on insufficient evidence.
23. Sayce has since described it, Proc. Roy. Soc. Victoria, xv., 1903, p. 229.
24. A. cancriformis had been supposed to have disappeared from the British fauna for many years, but it was found in Scotland in 1907. See R. Gurney, Nature, lxxvi., 1907, p. 589.
25. Branchipodides has been described by H. Woodward, from Tertiary strata.
26. Consult Baird, “Monograph of the Branchiopodidae,” Proc. Zool. Soc. 1852, p. 18. Packard, 12th Ann. Rep. U.S. Geol. Survey, part i., 1879.
27. Arch. f. Math. og Naturvidensk. xx., 1898, Nos. 4 and 6. Thiele, Zool. Jahrb. System. xiii., 1900, p. 563.
28. Bernard, loc. cit. p. 19; Baird, Proc. Zool. Soc. 1852, p. 1; Sayce, Proc. Roy. Soc. Victoria, xv., 1903, p. 224.
29. Sars, Arch. f. Math. og Naturvidensk. xx., 1898, Nos. 4 and 6.
30. Sars, Christiania Vidensk. Forhand. 1887. For Australian Phyllopods, see Sars, Arch. f. Math. og Naturvid. xvii., 1895, No. 7, and Sayce, loc. cit. p. 36.
31. Simocephalus vetulus anchors itself to weeds, etc., by a modified seta on the exopodite of the second antenna. It does not employ a dorsal organ for purposes of fixation. [G. S.]
32. Zeitschr. wiss. Zool. xxiv., 1874, p. 1.
33. Zeitschr. wiss. Zool. xxvii., xxxiii., 1876, 1879.
34. Consult Lilljeborg, Nov. Acta Reg. Soc. Upsalensis, 1901; Scourfield, J. Quekett Micr. Club, 1903–4.
35. More properly Chydoridae, but the universally known name Lynceidae is convenient.
36. Grundzüge der Zoologie, 4. Aufl. 1880, p. 543.
37. Fauna and Flora G. v. Neapel, Monograph 19, 1892.
38. Ibid. Monograph 25, 1899.
39. Norwegian North Polar Exp. Sci. Results, vol. i. part v., 1900.
40. They may assist the animal by retarding its sinking. Cf. Chun, “Aus den Tiefen des Weltmeeres,” 1905.
41. Schmeil, Bibliotheca Zoologica, Hefte 11, 15, and 21.
42. Giesbrecht, Mitth. Zool. Stat. Neap. xi., 1895, p. 648.
43. Loc. cit. p. 59.
44. Claus, Copepodenstudien, 1. Heft, Vienna, 1889.
45. Malaquin, Arch. Zool. Exp. (3), ix., 1901, p. 81.
46. Canu, Trav. Inst. Zool. Litte. vi., 1892.
47. Giesbrecht, Fauna and Flora G. v. Neapel, Monogr. 25, 1899.
48. Arb. Zool. Inst. Wien, ii. 1879, p. 268.
49. Ibid. xv., 1905, p. 1.
50. Heller, Reise der Novara, vol. iii., 1868.
51. For fish-parasites in British waters consult Scott, Fishery Board for Scotland, Scientific Investigations, xix., 1900 et seq.
52. Canu, loc. cit. p. 66.
53. The Cambridge Museum possesses two specimens of Philichthys xiphiae, from the frontal bones of a Swordfish (Xiphias gladius) taken off Lowestoft in 1892.
54. Claus, Arb. Zool. Inst. Wien, vii., 1888, p. 281.
55. Proc. Biol. Soc. Liverpool, i., 1887.
56. Zeitschr. wiss. Zool. xlix., 1890, p. 71.
57. The genus Pennella also includes parasites on the whales Hyperoodon and Balaenoptera.
58. Claus, Schriften d. Gesellsch. Marburg. Suppl. 1868.
59. Claus, Zeitschr. wiss. Zool. xi., 1861, p. 287.
60. Hansen, “The Choniostomatidae,” Copenhagen.
61. Claus, Zeitschr. wiss. Zool. xxv., 1875, p. 217.
62. C. B. Wilson, Proc. U.S. Nat. Museum, xxv., 1902, p. 635.
63. Max Müller (Science of Language, 2nd series, p. 534) gives references to a number of old authors who vouch for the truth of this legend, going back as far as Giraldus Cambrensis in the twelfth century. The legend appears to be of Scotch or Irish origin. Giraldus complains of the clergy in Ireland eating Barnacle geese at the time of fasting under the pretext that they are not flesh, but born of fish living in the sea. The form of the legend varies, certain authors alleging that the geese are produced from the fruits of a tree which drop into the water, others that they grow in shells (Barnacles) attached to floating logs. Aldrovandus (De Avibus, T. iii., 1603, p. 174) ingeniously combines both versions in a woodcut representing undoubted Barnacles growing on a tree with luxuriant foliage at the water’s edge, below which a number of liberated geese are swimming. Müller ascribes an etymological origin to the legend, the Barnacle goose (deriv. Hibernicula, bernicula = Irish goose) being confounded with pernacula, bernacula, a little shell.
64. “A Monograph of the Cirripedia,” vols. i. and ii., Ray Society, 1851, 1853.
65. “Rep. on the Cirripedia, H.M.S. ‘Challenger,’” vols. viii. and x., 1883.
66. “Monographie des Cirrhipèdes,” Paris, 1905, in which will be found full references to literature.
67. Arch. Biol. xvi., 1899, p 27.
68. Berndt, Sitzb. Ges. Naturfr. Berlin, 1903, p. 436.
69. Arch. Zool. Exp. viii., 1880, p. 537.
70. Quart. J. Micr. Sci. xxx., 1890, p. 107.
71. Plankton Expedition, ii. G. d. 1899.
72. Y. Delage, Arch. Zool. Exp. (2), ii., 1884, p. 417; G. Smith, Fauna u. Flora G. von Neapel, Monogr. 29, 1906.
73. G. Smith, Fauna u. Flora d. Golfes v. Neapel, Monogr. xxix., 1906, pp. 60–64, 119–121.
74. Bull. Sc. Dép. Nord (2), 10 Ann. xviii., 1887, p. 1. Ibid. (3), i., 1888, p. 12; and other papers.
75. G. Smith, loc. cit. chap. v. I. scorpio should be I. mauritanicus throughout this Monograph.
76. F. A. Potts, Quart. J. Micr. Sci. l., 1906, p. 599.
77. Faxon, Ann. Mag. Nat. Hist. (5), xiii., 1884, p. 147.
78. G. Smith, Mitth. Zool. Stat. Neapel, xvii., 1905, p. 312.
79. C. L. Boulenger, Proc. Zool. Soc. 1908, p. 42.
80. Garnier, C. R. Soc. Biol. liii., 1901, p. 38.
81. Gruvel, Monographie des Cirrhipèdes, 1905, p. 152.
82. Claus, Untersuchungen zur Erforschung des Crustaceensystems, Wien, 1876. Brady and Norman, “Monograph of the Marine and Fresh-Water Ostracoda of the N. Atlantic,” Trans. R. Dublin Soc. (2) iv., 1889, p. 63. Müller, Fauna und Flora G. von Neapel, Monogr. xxi., 1894; “Deutschlands Süsswasser-Ostracoden,” Chun’s Zoologica, xii., 1900.
83. “The Germ Plasm,” Contemp. Science Series, 1893, p. 345.
84. The term pereiopod is applied to those thoracic limbs which are used in locomotion, and are not specially differentiated for any other purpose.
85. Claus, Arb. Inst. Wien, viii., 1889, p. 1.
86. Robinson, Quart. J. Micr. Sci. 1., 1906, p. 383.
87. Morphol. Jahrb. viii., 1883, p. 485.
88. Ann. Mag. Nat. Hist. (7), xiii., 1904, p. 144.
89. The lacinia mobilis is a movable tooth-like structure jointed on to the biting face of the mandible.
90. Trans. Linn. Soc. (2), vi., 1894–1897, p. 285.
91. Trans. Roy. Soc. Edinburgh, xxxviii., 1897, p. 787.
92. G. Smith, Proc. Roy. Soc. 1908.
93. This characteristic is found in the Crustacea elsewhere only in the Argulidae and certain Euphausiidae.
94. The Victorian Naturalist, xxiv., 1907, p. 117.
95. Challenger Reports, vol. xiii., 1885, p. 55.
96. Sars, “Crustacea of Norway,” iii., 1900.
97. Sars, “Crustacea Caspia,” Bull. Acad. Imp. Sci. St. Pétersbourg, series 4, xxxvi., 1894, and “Crustacea of Norway,” iii., 1900, p. 120.
98. “Crustacea of Norway,” vol. ii., Isopoda, 1899, in which many references to literature will be found.
99. Smith, Mitth. Zool. Stat. Neapel, xvii., 1905, p. 312.
100. G. Smith, Mitth. Zool. Stat. Neapel, xvi., 1903, p. 469.
101. Mayer, Mitth. Zool. Stat. Neapel, i., 1879, p. 165.
102. Beddard, Challenger Reports, vol. xi., 1884.
103. Hansen, Quart. J. Micr. Sci. xlix., 1906, p. 69.
104. A useful little book on British Woodlice by Webb and Sillem (1906) may be profitably consulted. Budde Lund’s Isopoda Terrestria, 1900, is useful to the specialist.
105. The pleopods are traversed by a system of minute tubes called pseudotracheae, somewhat resembling the tracheae of Insects.
106. Bonnier, Trans. Inst. Zool. Lille, viii., 1900.
107. G. Smith, Fauna and Flora Neapel, Monograph 29, chap. vi.; M. Caullery, Mitth. Zool. Stat. Neapel, xviii., 1908, p. 583.
108. M. Caullery (loc. cit. p. 130) questions the truth of this observation, but I am convinced of its accuracy.
109. Trav. Inst. Lille, v., 1887.
110. Chilton, Trans. Linn. Soc. vi., 1894, p. 185.
111. Spenser and Hall, Proc. Roy. Soc. Victoria, ix. p. 12.
112. “Das Tierreich,” 21, Amphipoda Gammaridea, 1906.
113. Cf. P. Mayer, Fauna u. Flora G. von Neapel, Monogr. vi., 1882; xvii., 1890.
114. Abhandl. königl. Gesellsch. Göttingen, xvi., 1871.
115. Mem. Nat. Acad. Sci. v., 1891.
116. Sars, Challenger Reports, xiii., 1885; Chun, Bibliotheca Zoologica, xix., 1896, p. 139.
117. Die Physiologie der facettierten Augen von Krebsen und Insecten. Leipzig, Wien, 1891.
118. Valdivia Expedition, vol. vi., 1904.
119. Ann. Sci. Nat. (Zool.) (7), xiii., 1892, p. 185.
120. A Naturalist in Indian Seas, 1902.
121. “Atlantis,” Bibliotheca Zoologica, Heft 19, 1896, p. 193.
122. Loc. cit. p. 150.
123. Bell, A History of the British Stalk-eyed Crustacea, 1853; Heller, Die Crustaceen des Südlichen Europa, 1863.
124. Cf. Claus, Würzburger Naturwiss. Zeitschr. ii., 1861, p. 23.
125. Arch. f. Naturg. vi., 1840, p. 241.
126. Spence Bate’s Challenger Reports.
128. Bull. U.S. Fish Commission, xv., 1895.
129. Zool. Bulletin, i., 1898, p. 287.
130. Archiv für Entw. Mech. xi., 1901, p. 321.
131. Challenger Reports, xxiv., 1888.
132. Loc. cit. p. 150.
133. Keeble and Gamble, Phil. Trans., Ser. B, cxcvi., 1904, p. 295. The chromatophores are also directly responsive to light, but the lasting adaptations to colour-backgrounds are brought about indirectly, the stimulus being transmitted through the eyes and nervous system. The influence of light may also affect the metabolism of the animal, the chromatophores being accompanied by a ramifying fatty tissue, which disappears if the animal is kept in the dark.
134. Challenger Reports, xxiv., 1881.
135. Borradaile’s useful paper on the classification of the Decapoda (Ann. Mag. Nat. Hist. (7), xix., 1907, p. 457) should be consulted for this and other Decapod groups. Also Alcock’s Cat. of the Indian Mus., “Decapod Crustacea.”
136. Giard and Bonnier, Compt. Rend. Soc. Biol. 1892.
137. Coutière, Fauna and Geogr. Maldive and Laccadive Archipelagos, ii., 1905, p. 852.
138. Keeble and Gamble, Phil. Trans. Ser. B., cxcvi., 1904, p. 295. In the young a constant and very simple chromatophore-system is present, but in the adult a barred, lined, or monochrome colour-pattern may be present, which is ultimately induced by the nature of the environment, and does not subsequently change. In other species of Hippolyte, and in Palaemon and Crangon, only one adult colour-pattern occurs. Thus H. varians, besides reacting to light by its chromatophores, possesses a permanent colour-pattern, which is also determined by environment.
139. Claus, Unt. z. Erforschung d. genealog. Grundlage d. Crustaceensystems. Vienna, 1876.
140. Milne Edwards and Bouvier, Ann. Sci. Nat. (7), xvi., 1894, p. 91.
141. Garstang, Quart. J. Micr. Sci. xl., 1897, p. 211.
142. Milne Edwards and Bouvier, Bull. Soc. Philomath. Paris (8), ii., 1889; and Expédition du Talisman, “Crustacés Décapodes,” 1900.
143. Alcock, loc. cit.; Borradaile, op. cit. p. 162; i. p. 64.
144. Brandt, Bull. Phys. Math. Acad. St. Pétersbourg, i. p. 171, and viii. p. 54; Boas, K. Dansk. Vidensk. Selskab. Skrift. Naturvid. og Math. Afd. 6, Bd. 2, 1880; Bouvier, Ann. Sci. Nat. (Zool.) (7) xviii. p. 157.
145. Vol. xxvii. p. 81.
146. Proc. Boston Soc. Nat. Hist., xxxi., 1904, p. 147.
147. For general literature consult Ortmann in Bronn’s Tier-Reich, v. 2, 1901, p. 778. See also Reports of Challenger, Valdivia, and Talisman Expeditions, etc.
148. Gurney, Quart. J. Micr. Sci. xlvi., 1902, p. 461.
149. Bouvier, Bull. Soc. Philomath. Paris, (8) viii., 1896.
150. Loc. cit. p. 183.
151. M‘Culloch, Rec. Australian Mus. vi. part 5, 1907, p. 353.
152. Lankester, Quart. J. Micr. Sci. xlvii., 1903, p. 439.
153. Garstang, Quart. J. Micr. Sci. xl., 1897, p. 211, and Journ. Mar. Biol. Ass. iv., 1895–97, p. 396.
154. Loc. cit. p. 181.
155. Rep. Brit. Ass. for 1898, p. 887.
156. Naturalist in Indian Seas, 1902.
157. There appears to be some doubt on this point, as Westwood (see p. 153) described direct development in a Gecarcinus. Possibly different species behave variously.
158. Kingsley, Proc. Acad. Nat. Sci. Philadelphia, 1880, p. 187.
159. American Naturalist, xxxiii., 1899, p. 583.
160. Planktonstudien, Jena, 1890.
161. “Report on the Plankton,” Internat. Inst. Marine Biol. 1903.
162. Internat. Inst. Mar. Biol. 1903.
163. A Naturalist in Indian Seas.
164. Scourfield, J. Quekett Micr. Club, 1903–4, gives a useful list of British Fresh-water Entomostraca. For the identification of fresh-water Cladocera, Lilljeborg’s “Cladocera Sueciae,” Nov. Act. Reg. Soc. Upsalensis, 1901; for Copepoda, Schmeil’s “Süsswasser Copepoden,” in Bibliotheca Zoologica, iv., v., and viii., 1892, 1893, and 1895 are recommended.
165. Trans. Norfolk and Norwich Nat. Soc. vii.
166. Le Lac Leman, 3 vols., Lausanne, 1892.
167. Consult Apstein, “Das Süsswasserplankton,” Kiel and Leipzig, 1896; and Arch. f. Hydrobiologie u. Planktonkunde, numerous papers.
168. Mr. C. H. Martin points out to me that in the Scottish lochs, which from their geological nature are evidently not connected with subterranean waters, none of them nor similar forms occur; nor do they in the Tasmanian lakes which are on igneous diabase, so that Forel’s conclusion would seem to be of wide application.
169. See Chilton, Trans. Linn. Soc. (2) vi., 1894, p. 163, with review of literature.
170. S. F. Harmer, Trans. Norfolk and Norwich Nat. Soc. ii., 1899, p. 489.
171. Mem. Nat. Acad. Washington, iii., 1886, p. 1.
172. Arch. Zool. Exp. (4), ii., 1904, p. 1.
173. See Calman, Proc. Zool. Soc. 1906, p. 187.
174. The Crayfish, Internat. Scient. Series.
175. Mem. Harvard. Mus. x., 1885.
176. Proc. Amer. Phil. Soc. xli., 1902, p. 267, and xliv., 1905, p. 91.
177. G. O. Sars, “Crustacea Caspia,” Bull. Acad. Imp. Sc. St. Pétersbourg (4), xxxvi., 1893–4, pp. 51 and 297; (5) i., 1894, pp. 179 and 243; also Crustacea of Norway, vol. ii. Isopoda, 1900, p. 73.
178. Daday, Termés Füzetek, xxv., 1902, pp. 101 and 436.
179. Daday, Bibliotheca Zoologica, Heft 44, 1905.
180. On the cheek the furrow represents a pleural groove, and does not form the limit of the posterior cephalic segment.
181. M‘Coy, Synop. Sil. Foss. Ireland, 1846, p. 56, and Brit. Pal. Foss., 1851, p. 146, pl. 1 E, fig. 16; Salter, Quart. Journ. Geol. Soc. iii., 1847, p. 251.
182. Figures showing this suture are given by Oehlert, Bull. Soc. géol. de France (3), xxiii., 1895, pl. 1, figs. 9, 12, 15.
183. Ann. Mag. Nat. Hist. (2) iv., 1849, p. 396.
184. Lindström, “Visual Organs of Trilobites,” Svenska Vet. Akad. Handl. xxxiv., 1903. Exner, Physiol. d. facett. Augen v. Krebsen u. Insecten, 1891, p. 34, pl. ii. figs. 18, 19.
185. Journ. Morphol. ii., 1889, p. 253, pl. 21.
186. Watase, Johns Hopkins Univ. Studies, Biol. Lab. iv., 1890, p. 290. Lindström, op. cit. p. 27.
187. A suture is said to be present at the external margin of the flattened cephalic border.
188. Goldfuss, “Beitr. zur Petrefaktenkunde,” 1839, p. 359, pl. 33, fig. 2d.
189. Spencer, Geol. Mag. 1903, p. 489.
190. For an example of this see Salter, Mon. Brit. Trilobites, 1864–83, pls. 15, 16.
191. Bull. Mus. Comp. Zool. Harvard, viii., 1881, p. 191.
192. Studies in Evolution, 1901, pp. 197–225; Geol. Mag. 1902, p. 152. Walcott, Proc. Biol. Soc. Washington, ix., 1894, p. 89.
193. Syst. Sil. Bohême, i., 1852, pp. 257–276.
194. American Geologist, xx., 1897, p. 34.
195. Proc. R. Irish Acad. xxiv., 1903, p. 332, and Quart. Journ. Micr. Sci. xlix., 1906, p. 469.
196. This has received some support from H. Milne Edwards, Ann. Sci. Nat. Zool. (6), xii., 1881, p. 33; H. Woodward, Quart. Journ. Geol. Soc. xxvi., 1870, p. 487, and vol. 1., 1894, p. 433; Bernard, ibid. vol. 1. p. 432.
197. Kingsley does not admit this relationship, and regards the Trilobita as a group quite distinct from all other Crustacea. See American Naturalist, xxviii., 1894, p. 118, and American Geologist, xx., 1897, p. 33.
198. Zittel states that Apus appears first in the Trias.
199. Monogr. Brit. Trilobites, 1864, p. 2.
200. “A Natural Classification of Trilobites,” Amer. Jour. Sci. (4), iii., 1897, pp. 89–106, 181–207. Reprinted in Beecher’s Studies in Evolution, 1901, p. 109. A classification based on the character of the pygidium has been proposed by Gürich, Centralbl. für Min. Geol. u. Pal. 1907, p. 129. A classification based on the minute structure of the test has been given by Lorenz, Zeitschr. d. deutsch. geol. Gesellsch. lviii., 1906, p. 56.
201. Neues Jahrb. für Min. Geol. u. Pal. 1898, i. p. 187.
202. Lake, Brit. Cambrian Tril. 1907, p. 45.
203. The British Carboniferous Proëtidae are described by H. Woodward, Monogr. Brit. Carb. Trilobites, Palaeont. Soc. 1883–84.
204. This can be maintained in the Crustacea by counting the seventh abdominal segment, which appears in Gnathophausia; but this is not universally regarded as a true segment. See also Nebalia (p. 111).
205. This and the following Sub-class correspond with Lankester’s Sub-class Euarachnida. The Delobranchiata have gills patent and exposed, and adapted for breathing oxygen dissolved in water. The Embolobranchiata have either the gill-books (now termed lung-books) sunk into their body, or the gill-books are wholly or partially replaced by tracheae. In either case the members of this Sub-class breathe atmospheric oxygen.
206. Woodward, “On some Points in the Structure of the Xiphosura, having reference to their relationship with the Eurypteridae,” Quart. J. Geol. Soc. xxiii., 1867, p. 28, and xxviii., 1871, p. 46. Milne Edwards, A., “Recherches sur l’anat. des Limules,” Ann. Sci. Nat. (5), xvii., 1873, Art. 4. Lankester, E. R., “Limulus an Arachnid,” Quart. J. Micr. Sci. xxi., 1881, p. 504. Kingsley, J. S., “The Embryology of Limulus,” Journ. Morph. vii. p. 35, and viii. p. 195, 1892–3. Kishinouye, “On the Development of Limulus longispina,” Journ. Coll. Sci. Japan, v., 1892, p. 53. Patten, W., and Redenbaugh, W. A., “Studies on Limulus,” Journ. Morph. xvi., 1900, pp. 1, 91.
207. Quart. J. Micr. Sci. xlviii., 1905, p. 165.
208. μηρός = a thigh.
209. This segment, though present in embryo Scorpions, has disappeared in the adults of those animals.
210. Quart. J. Micr. Sci. xlix., 1906, p. 469.
211. Zool. Anz. xiv., 1891, pp. 164, 173.
212. Zeitschr. wiss. Zool. lix., 1895, p. 351.
213. They are described in great detail in Lankester’s article, “Limulus an Arachnid,” Quart. J. Micr. Sci. xxi., 1881, p. 504.
214. Tr. Linn. Soc. xxviii., 1872, p. 471.
215. Tr. Linn. Soc. xxviii., 1872, p. 472.
216. A rudimentary ninth pair of ostia are described anteriorly.
217. J. Morph. vii., 1892, p. 35.
218. Kingsley, loc. cit.
219. J. Coll. Tokyo, v., 1893, p. 53.
220. Lockwood, Amer. Nat. iv., 1870–71, p. 261.
221. For a diagnosis of the species and a list of synonyms, see Pocock, Ann. Mag. Nat. Hist. (7), ix., 1902, p. 256.
222. Quart. J. Micr. Sci. xxxi., 1890, p. 379; Proc. Cambr. Phil. Soc. ix., 1895–1898, p. 19; J. Anat. Physiol. xxxiii., 1899, p. 154.
223. Quart. J. Micr. Sci. xxxi., 1890, p. 317.
224. I am indebted to Mr. Henry Woods for these paragraphs on fossil Xiphosura.
225. The British fossil forms of this group are described and figured by H. Woodward, “Monograph of the Merostomata,” Palaeontogr. Soc. 1866–78, and Geol. Mag. 1907, p. 539.
226. Packard, “Carb. Xiphos. N. America,” Mem. Nat. Acad. Sci. Washington, iii., 1885, p. 146, pl. vi. fig. 1a, pl. v. fig. 3a (restoration). Williams, Amer. Journ. Sci. (3), xxx., 1885, p. 45. Fritsch, Fauna d. Gaskohle, iv., 1901, p. 64, pl. 155, figs. 1–3, and text-figures, 369, 370.
227. Walcott has described, under the generic name Beltina, imperfect specimens from the Algonkian (pre-Cambrian) of Montana, which he thinks may be the remains of Eurypterids (Bull. Geol. Soc. America, x., 1899, p. 238).
228. Walcott, Amer. Jour. Sci. (3), xxiii., 1882, p. 213.
229. Descriptions and figures of British Eurypterids are given in the following works:—Huxley and Salter, “Pterygotus,” Mem. Geol. Survey, Brit. Org. Remains, i., 1859; H. Woodward, “Monograph of the Merostomata,” Palaeont. Soc. 1866–78, and Geol. Mag. 1879, p. 196; 1887, p. 481; 1888, p. 419; 1907, p. 277; Peach, Trans. Roy. Soc. Edinb. xxx., 1882, p. 511; Laurie, ibid. xxxvii., 1892, p. 151; xxxvii., 1893, p. 509; and xxxix., 1899, p. 575.
230. A detailed account of Eurypterus fischeri has been given by G. Holm, Mém. Acad. Impér. Sci. St. Pétersbourg (8), viii. 2, 1898. See also F. Schmidt, ibid. (7), xxxi. 5, 1883. Descriptions of American forms of Eurypterus are given by Hall, “Nat. Hist. New York,” Palaeont. iii., 1859, p. 395; ibid. vii., 1888, p, 156; and Second Geol. Survey Pennsylvania, “Report of Progress,” PPP., 1884; Whiteaves, Geol. and Nat. Hist. Surv. Canada, “Palaeozoic Foss.,” iii., 1884, p. 42.
231. It was this ornamentation found on fragments of Pterygotus anglicus which led the Scotch quarrymen to apply the name “Seraphim” to that Eurypterid. On this subject Hugh Miller writes: “The workmen in the quarries in which they occur, finding form without body, and struck by the resemblance which the delicately waved scales bear to the sculptured markings on the wings of cherubs—of all subjects of the chisel the most common—fancifully termed them ‘Seraphim’” (The Old Red Sandstone, ed. 6, 1855, p. 180).
232. The third leg in the male possesses on the fifth joint a curved appendage which extends backwards to the proximal end of the second joint. This structure may have been a clasping organ.
233. It has been suggested that the metastoma really belongs to a pregenital segment of the mesosoma which is absent in the adult, but has been found in the embryo of Scorpions.
234. Sarle, New York State Museum, Bulletin 69, Palaeont. 9, 1903, p. 1087.
235. Beecher, Geol. Mag. 1901, p. 561.
236. Peach, Nature, xxxi., 1885, p. 295; Pocock, Quart. Journ. Micr. Sci. xliv., 1901, p. 291; Laurie, Trans. Roy. Soc. Edinb. xxxix., 1899, p. 575.
237. Peach, Trans. Roy. Soc. Edinb. xxx., 1882, p. 516.
238. Cf. p. 258.
239. Nature, xlviii., 1893, p. 104.
240. Souvenirs entomologiques, Sér. 9, 1907, p. 229.
241. Brauer, Zeitschr. wiss. Zool. lix., 1895, p. 355.
242. Das Tierreich, 8. Lief., 1899, p. 4.
243. Arachnides de France, vii., 1879, p. 84.
244. Fauna of British India, “Arachnida,” 1900, p. 8.
245. Tr. Zool. Soc. xi. part x., 1885, p. 373.
246. Zeitschr. wiss. Zool. lix., 1895, p. 351.
247. Das Tierreich, 8. Lief., 1899.
248. Pocock, Fauna of British India, “Arachnida.” London, 1900.
249. Laurie, J. Linn. Soc. Zool. xxv., 1894, p. 30.
250. See M. Laurie in J. Linn. Soc. Zool. xxv., 1894, p. 20.
251. Tr. Linn. Soc. (2) vi., 1896, p. 344.
252. Bernard, loc. cit. p. 366.
253. J. Linn. Soc. xxv., 1894, p. 29.
254. See Pocock, Ann. Nat. Hist. (6), xiv., 1894, p. 120.
255. Kraepelin, Das Tierreich, Berlin, 8. Lief., 1899, p. 234.
256. The term mostly in use is Araneida, which should mean Araneus-like animals. This is clearly not allowable, unless there is a genus Araneus or Aranea. For many years there has been no such genus recognised, but Simon now attempts to re-establish it, inadmissibly, as it appears to us. (See note, p. 408).
257. Mém. Mus. d’Hist. Nat. xviii., 1829, p. 377.
258. Pickard-Cambridge (Spiders of Dorset, 1879–1881) omits the coxal joint, which, with its lobe, he calls the maxilla, and therefore gives only five joints, which he names axillary, humeral, cubital, radial, and digital.
259. Pickard-Cambridge, in his Spiders of Dorset, names them exinguinal, coxal, femoral, genual, tibial, metatarsal, and tarsal.
260. Nat. Hist. Tidsskr. iv., 1843, p. 349.
261. J. Linn. Soc. xv., 1881, p. 155.
262. Proc. Asiat. Soc. Beng. 1875, p. 197.
263. Tijdschr. v. d. Nederl. Dierkundige Ver. (2), i., 1885–1887, p. 109.
264. Études sur la circulation du sang chez les Aranées du genre Lycose. Utrecht, 1862.
265. Recherches sur l’appareil circulatoire des Aranéides. Lille, 1896.
266. Arch. f. Naturg. 55 Jahrg., i., 1889, p. 29.
267. M‘Leod, Bull. Ac. Belg. (3), iii., 1882, p. 779.
268. American Spiders and their Spinning Work, ii., 1890, p. 208.
269. Ann. Nat. Hist. (3), xv., 1865, p. 459.
270. Voyage of the Beagle.
271. Correspondence of John Ray, p. 77.
272. Warburton, Q. J. Micr. Sci. xxi., 1890, p. 29.
273. Rep. Brit. Ass. 1844, p. 77.
274. Nature, xl., 1889, p. 250.
275. See Warburton, Quart. J. Micr. Sci. xxi., 1890, p. 29.
276. Aranéides de la Réunion, Maurice et Madagascar, Paris, 1863, p. 238.
277. M‘Cook, American Spiders and their Spinning Work, i., 1889, p. 351; F. O. Pickard-Cambridge, J. Micr. and Nat. Sci. July 1890.
278. Moggridge, Harvesting Ants and Trap-door Spiders. London, 1873, p. 120.
279. Verh. Ges. Wien, xviii., 1868, p. 905 (Abstract in Zool. Rec. v., 1868, p. 175).
280. The figure of this cocoon has been accidentally inverted in the works of both Blackwall and Pickard-Cambridge.
281. Fabre, Nouveaux souvenirs entomologiques, ch. xi.
282. Aranéides de la Réunior, Maurice et Madagascar, Paris, 1863, p. xlvi.
283. Hist. de la grande île de Madagascar, 1658, p. 156.
284. Science Gossip, 1877, p. 46.
285. Insect Life, i., 1889, p. 205.
286. Ann. Soc. ent. France, xi., 1842, p. 205. Translated from the Spanish by L. Fairmaire.
287. M‘Cook, American Spiders and their Spinning Work, ii., 1890, p. 188.
288. M‘Cook, t.c. p. 389.
289. British Spiders, 1861, p. 102.
290. Ann. Nat. Hist. (1), xi., 1843, p. 1.
291. Naturalist in Nicaragua, 2nd ed., 1888, p. 134.
292. Nouveaux souvenirs entomologiques, ch. xii.
293. M‘Cook, t.c. p. 384.
294. The Naturalist in Nicaragua, p. 19.
295. Spiders of Dorset, 1879–1881, p. 292.
296. Ibid. p. 360.
297. Naturalist on the Amazon, 1873, p. 54.
298. Protective Resemblances and Mimicry in Animals, 1873, p. 4.
299. Nature, lxviii., 1908, p. 631.
300. J. Morph. (Boston, U.S.A.) i., 1887, p. 403.
301. Nature, xxiii., 1880, p. 149.
302. Warburton, Ann. Nat. Hist. (6), viii., 1891, p. 113.
303. Spiders of Dorset, 1879–1881, p. xxvii.
304. Spiders, their Structure and Habits, 1883, p. 98.
305. Sexual Selection in Spiders, p. 37. (Occasional Papers of the Nat. Hist. Soc. of Wisconsin, I., 1889.)
306. Arachnides de France (vol. i., published 1874). Histoire naturelle des araignées (2nd ed. vol i., published 1892).
307. Simon’s Cribellatae comprise Hypochilidae, Uloboridae, Psechridae, Zoropsidae, Dictynidae, Oecobiidae, Eresidae, Filistatidae.
308. The Spider genus Mygale was established by Walckenaer in 1802, but the name was preoccupied, having been used by Cuvier (Mammalia) in 1800.
309. Hist. Nat. des Araignées (2nd ed.), i., 1892, p. 76.
310. The “scopula” is the pad of close-set thick hairs which covers the under surface of the tarsus and often of the metatarsus. The “claw-tufts” are groups of longer hairs, often extending beyond the claws, and giving the foot a bifid appearance.
311. The three families mentioned above constitute the “Araneae Theraphosae” of Simon, the remaining families being distinguished as “Araneae Verae.” The Aviculariidae and the Atypidae are united by some authors to form the Theraphosidae.
312. According to Bertkau (in a letter to Simon, cited in Hist. Nat. des Ar. i. p. 327), two pairs of linear stigmata under the anterior part of the abdomen lead, to pulmonary sacs, but to tracheae.
313. L. Koch replaced Melanophora by Prosthesima, believing the former to be preoccupied, but according to Simon (Hist. Nat. des Ar. i. p. 341) C. Koch’s use of Melanophora for an Arachnid was antecedent (1833) to Meigen’s employment of it for Diptera, 1838.
314. Hist. Nat. des Ar. i. p. 416.
315. Pickard-Cambridge, Spiders of Dorset, p. 77.
316. Hist. Nat. des Ar. i. p. 594.
317. Hist. Nat. des Ar. i. p. 692.
318. The Erigoninae, Formicinae, and Linyphiinae, together with the Epeiridae, form Simon’s family of Argiopidae.
319. I.e. as developed in the course of the work, not as set forth on p. 594 of vol. i., where five sub-families are established (Theridiosomatinae, Arciinae, Eurycorminae, Amazulinae, Poltyinae), which are afterwards merged in the Argiopinae.
320. Simon’s treatment of this group in his Hist. Nat. Ar. does not appear to us satisfactory. He revives the name Araneus as a generic term, a proceeding to which there are very valid objections, and merges in it, in whole or in part, about twenty-five generally received genera, including 800 species. He then proceeds to break up the genus Araneus into six entirely artificial “series,” according to the eyes. However unsatisfactory the merged genera may be, nothing seems to be gained by this proceeding. The facts about “Araneus” are these. Clerck and Linnaeus used the name “Araneus” for every member of the order. Latreille, in subdividing the order, retained the name for A. (Epeira) diademata (1804), but later (1827) transferred it to A. (Tegenaria) domestica. Walckenaer, seeing the impropriety of using Araneus as a generic term, discarded it, establishing Epeira, which has since obtained universal recognition.
321. Simon, in his Histoire naturelle des araignées, removes the Sparassinae and the Selenopinae to the Clubionidae, considering that, notwithstanding the direction of their legs, they have a greater affinity with that group than with the other Thomisidae.
322. Ent. Tidsskr. xviii., 1897, p. 223, pl. iv.
323. Zool. Anz. xxiv., 1901, p. 537.
324. Quart. J. Micr. Sci. xlvii., 1904, p. 215.
325. Trans. Linn. Soc. (2), vi., 1896, p. 323.
326. Nature, xlvi., 1892, p. 247.
327. Ann. Nat. Hist. (1), xii., 1843, p. 81.
328. Pocock, Nature, lvii., 1897, p. 618.
329. Cook, Nature, lviii., 1898, p. 247.
330. Öfv. Ak. Förh. lvi., 1899, p. 977.
331. Trans. Linn. Soc. (2), vi., 1896, p. 310.
332. Das Tierreich, Berlin, 12. Lief., Arachnoidea, 1901, p. 4.
333. Arachnides de France, vii., 1879, p. 2.
334. Arachnides de France, vii., 1879, p. 5.
335. See Bernard, J. Linn. Soc. xxiv. (Zool.), 1893, p. 410.
336. See Bernard, J. Linn. Soc. xxiv. (Zool.), 1893, p. 422.
337. For the embryology of Chernetidea, see J. Barrois, “Mém. sur le développement des Chélifers,” Rev. Suisse de Zool. iii., 1896. Metschnikoff, Zeitschr. wiss. Zool. xxi., 1876, p. 514; and Vejdovský, Congrès zool. international de Moscou, 1892, p. 120, may also be consulted.
338. Monograph of the British Species of Chernetidea, Dorchester, 1892.
339. Revue Zoologique par la Société Cuvierienne, p. 10.
340. Arachnides de France, vii., 1879, p. 122.
341. On two Orders of Arachnida, Cambridge University Press, 1904.
342. Mag. Nat. Hist. (i.), xii., 1843, p. 325.
343. Zool. Jahrb. iii., 1888, p. 319.
344. T. C. pp. 67–75.
345. Long sternum (μῆκος = length; στῆθος = breast).
346. Arachnides de France, vii., 1879.
347. Transverse sternum (πλάγιος = transverse).
348. Monograph of the British Phalangidea, Dorchester, 1890.
350. C. R. Ac. Sci. cxxv., 1897, p. 879.
351. “The Biology of the Cattle Tick,” Journ. Compar. Med. and Vet. Archives, 1891, p. 313.
352. Entomological News (Philadelphia), vol. xi., Jan. 1900.
353. For the Protozoa to which these and similar diseases are due, cf. vol. i. pp. 120 f.
354. C. R. Soc. Biol. Paris (7), iv., 1882, p. 305.
355. Ann. Soc. Linn. Lyon, xxii., 1876, p. 29.
356. Bull. Soc. Nat. de Moscou, liv. 1879, pt. i. p. 234.
357. Z. wiss. Zool. xxxvii., 1882, p. 553.
358. P. Z. S., 1895, p. 174.
359. Arch. f. Naturg. i., 1876, p. 65.
360. Tr. Linn. Soc. (2), v. Zool., 1890, p. 281.
361. See account given by Tulk in Mag. Nat. Hist. xviii., 1846, p. 160.
362. Entomological News (Philadelphia), vol. xi., Jan. 1900.
363. Michael, British Oribatidae (Ray Soc.), i., 1883, p. 176.
364. Loc. cit. p. 168.
365. Claparède, Z. wiss. Zool. xviii., 1868, p. 455. Michael, British Oribatidae, i., 1883, p. 73, writes it “Deutovium.”
366. Atti Ist. Veneto, ii., 1891, p. 699.
367. Rev. Sci. Nat. Ouest, ii., 1892, p. 20.
368. Eriophyes, v. Siebold, Jahresber. Schles. Ges. xxviii., 1850, p. 89; Phytoptus, Dujardin, Ann. Sci. Nat. (3), xv., 1851, p. 166.
369. See Michael, British Tyroglyphidae, published by the Ray Society, 1901–2.
370. The first paper appeared in Mém. Soc. Zool. ix., 1896, pp. 1–44.
371. “Ticks, a Monograph of the Ixodoidea.” Part I. Argasidae, 1908.
372. With, Vid. Medd. 1904, p. 137.
373. Silvestri, Redia, ii., 1904, fasc. 2, p. 257.
374. Arch. mikr. Anat. Bd. i., 1865, p. 428.
375. A. Basse, Zeitschr. wiss. Zool. lxxx., 1906, p. 259.
376. Ann. Sci. nat. (2), xiv., 1840, p. 269, and xvii., 1842, p. 193.
377. Zool. Jahrb. Anat. iii., 1889. This paper contains a bibliography.
378. Morph. Jahrb. xxii., 1895, p. 491.
379. C. R. Ac. Sci. cxviii., 1894, p. 817.
380. Tr. R. Soc. Edinb. xlv., 1908, p. 641. This contains a Bibliography of recent literature. See also Richters, Zool. Anz. xxx., 1906, p. 125, and Heinis, Zool. Anz. xxxiii., 1908, p. 69.
381. P. Zool. Soc. 1897, p. 790.
382. Hay, in P. Biol. Soc. Washington, xix., 1906, p. 46, states that the name Lydella, Dujardin, is preoccupied, and suggests as a substitute Microlyda.
383. The animals included in this group are usually called Linguatulidae or Pentastomidae after the two genera or sub-genera Linguatula and Pentastoma. But the animal which Rudolphi in 1819 (Synopsis Entozoorum) named Pentastoma had been described, figured, and named Porocephalus by Humboldt (Recueil d’observations de zoologie et anatomie comparee, i. p. 298, pl. xxvi.) in 1811. The familiar name Pentastoma may, however, be preserved by incorporating it in the designation of the group.
384. This description is mainly based on the account of P. teretiusculus given by Spencer, Quart. J. Micr. Sci. xxxiv., 1893, p. 1.
385. Zeitschr. wiss. Zool. lii., 1891, p. 85. This contains a very full bibliography, of 143 entries.
386. Centrbl. Bakter. xl., 1906, p. 368; v. also Thiroux, C. R. Soc. Biol. lix., 1905, p. 78.
387. Shipley, Arch. parasit. i., 1898, p. 52. This contains lists of synonyms and of memoirs published since Stiles’ paper, etc.
388. H. B. Ward, P. Amer. Ass. 1899, p. 254.
389. Nouv. Dict. de méd., de chir. et d’hyg. vétérinaires, xii. 1883.
390. Tr. R. Soc. Edinb. xxxii., 1884, p. 165.
391. Lohrmann, Arch. Naturg. Jahrg. 55, i., 1889, p. 303.
392. Von Linstow, J. R. Asiat. Soc. Bengal, ii., 1906, p. 270.
393. Pycnogonides, Latreille, 1804; Podosomata, Leach, 1815; Pychnogonides ou Crustacés aranéiformes, Milne-Edwards, 1834; Crustacea Haustellata, Johnston, 1837; Pantopoda, Gerstaecker, 1863.
394. Syst. Nat. ed. xii. 1767, vol. ii. p. 1027.
395.
Brünnich’s description (“Entomologia,” 1764), is still more accurate, and is worthy of transcription as an excellent example of early work. “Fig. iv. Novum genus, a R[ev.] D[on.] Ström inter phalangiis relatum, Söndm. Tom. i. p. 209, t. 1, f. 17. Exemplar hujus insecti, quod munificentia R. Autoris possideo, ita describo; Caput cum thorace unitum, tubo b excavato cylindrico, antice angustiore, postice in thoracem recepto, prominens; Oculi iv. dorsales, a, in gibbositate thoracis positi; c, Antennae 2 tubo breviores moniliformes, subtus in segmento thoracis, cui oculi insident, radicatae; segmenta corporis, excepto tubo, iv., cum tuberculo e medio singuli segmenti prominulo. Pedes viii., singuli ex articulis vii. brevissimis compositi, ungue valido terminati. Ex descriptione patet insectum hoc a generibus antea notis omnino differre, ideoque novum genus, quod e crebris articulationibus Pycnogonum dico, constituit.” The confusion between Cyamus and Pycnogonum seems to have arisen with Job Baster, 1765; cf. Stebbing, Knowledge, February 1902, and Challenger Reports, “Amphipods,” 1888, pp. 28, 30, etc.
396. Hoek, Chall. Rep. p. 15, mentions a specimen of Colossendeis gracilis, Hoek, “furnished with a pair of distinctly three-jointed mandibles; and the specimen was the largest of the three obtained.”
397. As a rare exception, Hoek has found the eggs carried on the ovigerous legs in a single female of Nymphon brevicaudatum, Miers.
398. Meisenheimer (Zeitsch. wiss. Zool. lxxii., 1902, p. 235) compares these with certain glands described in Branchipus by Spangenberg and by Claus.
399. Ortmann, who would unite Barana with Ascorhynchus, observes: “Bei dieser Gattung [Ascorhynchus] konnte ich die Kittdrüsen beobachten, die bei A. ramipes mit dem von Barana castelnaudi [castelli] Dohrn, bei A. cryptopygius mit Barana arenicola übereinstimmen und also die primitivsten Formen der Ausbildung zeigen.”—Zool. Jahrb. Syst. v., 1891, p. 159.
400. Mém. Acad. Sci. St-Pétersb. (vii.), xxxviii., 1892.
401. Fauna und Flora G. von Neapel, iii. Monogr. 1881, p. 46; see also Loman, J. C. C., Tijdschr. D. Ned. Dierk. Ver. (2), viii., 1907, p. 259.
402. The dorsal lobe is absent in Rhynchothorax.
403. For a very detailed account of this mechanism, here epitomised in the merest outline, and for an account of its modifications in diverse forms, the student must consult Dohrn’s Monograph (t. cit. pp. 46–53).
404. Dohrn, t. cit. p. 55.
405. Biol. Stud. Johns Hopkins Univ. v., 1891, p. 49.
406. Vergl. Entwickl. d. wirbellosen Tiere, Jena, 1893, p. 664.
407. In the second joint in Ascorhynchus abyssi, Sars, and A. tridens, Meinert.
408. Biol. Bulletin Woods Holl, vol. ii., Feb. 1901, p. 196.
409. Studi e ricerche sui Picnogonidi, Firenze, 1876.
410. Semper came near to discovering the fact when he saw, at Heligoland, ripe eggs in a Phoxichilidium that was, nevertheless, totally destitute of ovigerous legs. The animal, he says, was adult and sexually mature: “Trotzdem fehlen dem Tiere die Eierträger vollständig; es muss sich also das Tier noch mindestens ein Mal häuten vor der Eierablag, und dabei müssen die Eierträger gebildet werden.” (Arb. Inst. Würzburg, 1874, p. 273).
411. The correspondence is not universally admitted. Meinert (Ingolf Expedition, 1899) believes that the second and third appendages of the larva disappear, and that the palps and ovigerous legs are new developments; so giving to the normal Pycnogon nine instead of seven appendages. See also Carpenter “On the Relationship between the Classes of the Arthropoda,” Proc. R. Irish Acad. xxiv., 1903, pp. 320–360. The latest observer (Loman) inclines to the older view.
412. A slightly different account is given of the Australian P. plumulariae by v. Lendenfeld (Zeitschr. wiss. Zool. xxxviii., 1883, pp. 323–329).
413. Zur Lehre vom Generationswechsel und Fortpflanzung bei Medusen und Polypen, 1854.
414. Rep. Brit. Ass. 1859; cf. “Gymnoblastic Hydroids,” Ray Soc. pl. vi. fig. 6.
415. Trans. Tyneside Field Club, v. (1862–3), 1864, pp. 124–136, pls. vi., vii.; Ann. Mag. Nat. Hist. (3), ix., 1862, p. 33.
416. See also Hallez, Arch. Zool. Exp. (4), v., 1905, p. 3; Loman, Tijdschr. Ned. Dierk. Ver. (2), x., 1906, p. 271, etc.
417. “On Hydroid and other Corals,” 1881, p. 78.
418. Hugo Mertens, Mitth. Zool. Stat. Neapel, xviii., 1906, pp. 136–141.
419. One is tempted to explain such cases as the above of harmonious or identical coloration by the simple passage of pigments unchanged from the food.
420. Fabricius says of his Pycnogonum (Nymphon) grossipes, “Vescitur insectis et vermibus marinis minutis; quod autem testas mytilorum exhauriat mihi ignotum est, dum nunquam intra testam mytili illud inveni, licet sit verisimile satis,” Fauna Groenlandica, p. 231.
421. Loeb (Arch. Entw. Mech. v. 2, 1897, p. 250) also says that the Pycnogons are positively heliotropic.
422. See also P. Gaubert, “Autotomie chez les Pycnogonides,” Bull. Soc. Zool. Fr. xvii., 1892, p. 224.
423. Cf. Carpenter, Proc. R. Irish Acad. xxiv., 1903, p. 320; Lankester, Quart. J. Micr. Sci. xlviii., 1904, p. 223; Bouvier, Exp. Antarct. Fr., “Pycnogonides,” 1907, p. 7, etc.
424. “Nous ne les plaçons ici qu’avec doute,” Règne Anim. éd. 3, tom. vi. p. 298.
425. Cf. also J. E. W. Ihle, “Phylogenie und systematische Stellung der Pantopoden,” Biol. Centralbl., Bd. xviii., 1898, pp. 603–609; Meisenheimer, Verh. zool.-bot. Ges. Wien, xii., 1902, pp. 57–64; also Stebbing, in Knowledge, 1902.
426. The chelate form of the foremost appendages is of little moment. A chela consists merely of a more or less mobile terminal joint flexing on a more or less protuberant penultimate one, and in the Scorpions, in Limulus, throughout the Crustacea, and even in Insects (cf. vol. vi. p. 554), we see such a structure arising independently on very diverse appendages.
427. Cf. Oudemans, Tijdschr. d. Ned. Dierk. Ver. (2), i., 1886, p. 41: “Jedermann weiss nun, dass diese Tiere eine ganz besondere Urgruppe bilden, ohne alle Verwandschaft mit irgend einer anderen Arthropodengruppe.”
428. Cole (Ann. Mag. Nat. Hist. (7), xv., 1905, pp. 405–415) has attempted such a phylogenetic classification, starting with Decolopoda, and leading in two divergent lines, through Nymphon and Pallene to the Pycnogonidae, and through Eurycide and Ammothea to Colossendeis. This hint is in part adopted in the subjoined classification. Bouvier, in his recent Report on the Pycnogons of the French Antarctic Expedition (t. cit.), gives reasons for separating the Decolopodidae and Colossendeidae from all the rest. Loman, in Die Pantopoden der Siboga Expedition, 1908, has recently suggested another, and in many respects novel, classification of the whole group.
429. See (inter alia) Dohrn, l.c.; E. B. Wilson, Rep. U.S. Fish. Comm. (1878), 1880; Hoek, Chall. Report, 1881; G. O. Sars, Norw. N. Atl. Exp. 1891; Meinert, Ingolf Exped. 1899; Möbius, Fauna Arctica, 1901, Valdivia Exped. 1902; Cole, Harriman Alaska Exped. 1904; Hodgson, Discovery Exped. 1907; Bouvier, Exp. Antarct. Fr. 1907.
430. Boston Journ. Nat. Hist. i., 1834, p. 203; Cf. Hodgson, Pr. R. Phys. Soc. Edinburgh, xvi., 1905, p. 35; Zool. Anz. xxv., 1905, p. 254; Discovery Exp., “Pycnogonida,” 1907; Bouvier, Exp. Antarct. Fr. 1907.
432. The first known species was described as Phoxichilus proboscideus, Sabine, from the shores of the North Georgian Islands (1821).
433. Pocock (Encycl. Brit., 10th ed., Art. “Arachnida”) makes Hannonia the solitary type of a family. Cf. Loman, Zool. Jahrb., Syst., xx., 1904, p. 385.
434. Loman conjoins all these genera, and also Lecythorhynchus, with Nymphopsis, as a sub-family Nymphopsinae of Ammotheidae.
435. Edinb. New Phil. Journal, Oct. 1842, p. 367 (P. capillata on Plate).
436. Proc. Boston Nat. Hist. Society, vol. i., 1841–44, p. 92.
437. Found by Sir John Ross’s expedition in 1840, and subsequently by the Challenger expedition and other visitors.
438. Stebbing has recently shown (Knowledge, Aug. 1902, p. 157) that the genus Phoxichilus was instituted by Latreille (Nouv. Dict. d’hist. nat. 1804) for the Pycnogonum spinipes of Fabricius, now Pseudopallene spinipes, auctt. Hence he changes Pseudopallene to Phoxichilus, Latr., and Phoxichilidae and Phoxichilus, auctt., to Chilophoxidae, etc.; it also follows that the family known to all naturalists as Pallenidae should, according to the letter of the law of priority, be henceforth known as the Phoxichilidae. In my opinion this is a case where strict adherence to priority would serve no good end, but would only lead to great and lasting confusion (cf. Norman, J. Linn. Soc. xxx., 1908, p. 231).
439. Vide note 2, p. 537.
440. Mag. Nat. Hist. vi., 1838, p. 42; Mag. Zool. and Bot. i., 1837, p. 368.
441. Edinb. New Phil. Journ. xxxii., 1842, p. 136; xxxiii., 1842, p. 367; Ann. Mag. Nat. Hist. (1), xiv., 1844, p. 4.
442. Ann. Mag. Nat. Hist. (3), xiii., 1864, p. 113.
443. Proc. R. Dublin Soc. (N.S.), viii., 1893, p. 195; Fisheries, Ireland, Sci. Invest. 1904, No. iv. (1905).
444. Cf. A. M. Norman, J. Linn. Soc. xxx., 1908, pp. 198–238.
FIELD.—“The Cambridge Natural History series of volumes is one of very great value to all students of biological science. The books are not intended for popular reading, but for utilisation by those who are desirous of making themselves thoroughly acquainted with the branches of zoology of which they treat.”
Protozoa, by Marcus Hartog, M.A., D.Sc.; Porifera (Sponges), by Igerna B. J. Sollas, B.Sc.; Coelenterata and Ctenophora, by S. J. Hickson, M.A., F.R.S.; Echinodermata, by E. W. MacBride, M.A., F.R.S.
FIELD.—“The book can be in the strongest manner recommended to those for whose benefit it has been written. We know of no work from which a more truly scientific account of the Protozoa, Echinodermata, and other lower forms of animal life could be gained.”
Flatworms and Mesozoa, by F. W. Gamble, D.Sc.; Nemertines, by Miss L. Sheldon; Threadworms and Sagitta, by A. E. Shipley, M.A., F.R.S.; Rotifers, by Marcus Hartog, M.A., D.Sc.; Polychaet Worms, by W. Blaxland Benham, D.Sc., M.A.; Earthworms and Leeches, by F. E. Beddard, M.A., F.R.S.; Gephyrea and Phoronis, by A. E. Shipley, M.A., F.R.S.; Polyzoa, by S. F. Harmer, Sc.D., F.R.S.
CAMBRIDGE REVIEW.—“Most of the articles are of a very high order of merit—taken as a whole, it may be said that they are by far the best which have as yet been published.... We may say with confidence that the same amount of information, within the same compass, is to be had in no other zoological work.”
Molluscs, by the Rev. A. H. Cooke, M.A.; Brachiopods (Recent), by A. E. Shipley, M.A., F.R.S.; Brachiopods (Fossil), by F. R. C. Reed, M.A.
TIMES.—“There are very many, not only among educated people who take an interest in science, but even among specialists, who will welcome a work of reasonable compass and handy form containing a trustworthy treatment of the various departments of Natural History by men who are familiar with, and competent to deal with, the latest results of scientific research. Altogether, to judge from this first volume, the Cambridge Natural History promises to fulfil all the expectations that its prospectus holds out.”
Crustacea, by Geoffrey W. Smith, M.A., and the late W. F. R. Weldon, M.A.; Trilobites, by Henry Woods, M.A.; Introduction to Arachnida, and King-Crabs, by A. E. Shipley, M.A., F.R.S.; Eurypterida, by Henry Woods, M.A.; Scorpions, Spiders, Mites, Ticks, etc., by Cecil Warburton, M.A.; Tardigrada (Water-Bears), by A. E. Shipley, M.A., F.R.S.; Pentastomida, by A. E. Shipley, M.A., F.R.S.; Pycnogonida, by D’Arcy W. Thompson, C.B., M.A.
Peripatus, by Adam Sedgwick, M.A., F.R.S.; Myriapods, by F. G. Sinclair, M.A.; Insects, Part I., Introduction, Aptera, Orthoptera, Neuroptera, and a portion of Hymenoptera (Sessiliventres and Parasitica), by David Sharp, M.A., M.B., F.R.S.
Prof. RAPHAEL MELDOLA, F.R.S., F.C.S., in his Presidential Address to the Entomological Society of London, said:—“The authors of this volume are certainly to be congratulated upon having furnished such a valuable contribution to our literature. When its successor appears, and I will venture to express the hope that this will be at no very distant period, we shall be in possession of a treatise on the natural history of insects which, from the point of view of the general reader, will compare most favourably with any similar work that has been published in the English language.”
Hymenoptera (continued) (Tubulifera and Aculeata), Coleoptera, Strepsiptera, Lepidoptera, Diptera, Aphaniptera, Thysanoptera, Hemiptera, Anoplura, by David Sharp, M.A., M.B., F.R.S.
SATURDAY REVIEW.—“Dr. Sharp’s treatment is altogether worthy of the series and of his own high scientific reputation.... Certainly this is a book that should be in every entomologist’s library.”
Hemichordata, by S. F. Harmer, Sc.D., F.R.S.; Ascidians and Amphioxus, by W. A. Herdman, D.Sc., F.R.S.; Fishes (exclusive of the Systematic Account of Teleostei), by T. W. Bridge, Sc.D., F.R.S.; Fishes (Systematic Account of Teleostei), by G. A. Boulenger, F.R.S.
ATHENÆUM.—“All who take a serious interest in the advance of ichthyology will find this a fascinating book.”
Amphibia and Reptiles, by Hans Gadow, M.A., F.R.S.
NATURE.—“In concluding the review we would express the opinion that by this handsome volume a very important addition to science has been made; that the beautiful illustrations, together with the clear and charming accounts of the life-histories which it contains, will do much to popularise the study of a rather neglected section of zoology; and that lovers of Reptiles, of which there are more than one generally thinks, will feel that the new knowledge imparted to them emanates from one who is thoroughly in sympathy with their enthusiasm.”
Birds, by A. H. Evans, M.A.
IBIS.—“Mr. Evans has produced a book full of concentrated essence of information on birds, especially as regards their outer structure and habits, and one that we can cordially recommend as a work of reference to all students of ornithology.”
Mammalia, by Frank Evers Beddard, M.A., F.R.S.
NATURE.—“Cannot fail to be of very high value to all students of the Mammalia, especially from the standpoints of morphology and palæontology.”
Retrieved from "http://en.wikipedia.org/"
All text is available under the terms of the GNU Free Documentation License

