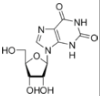
Nucleobases (or nucleotide bases/nitrogenous bases) are the parts of DNA and RNA that may be involved in pairing (see also base pairs). The primary nucleobases are cytosine, guanine, adenine (DNA and RNA), thymine (DNA) and uracil (RNA), abbreviated as C, G, A, T, and U, respectively. They are usually simply called bases in genetics. Because A, G, C, and T appear in the DNA, these molecules are called DNA-bases; A, G, C, and U are called RNA-bases. Uracil replaces thymine in RNA. These two bases are identical except that uracil lacks the 5' methyl group. Adenine and guanine belong to the double-ringed class of molecules called purines (abbreviated as R). Cytosine, thymine, and uracil are all pyrimidines (abbreviated as Y). The system of a base covalently bound to the 1' carbon of a ribose or deoxyribose is called a nucleoside, and a nucleoside with one or more phosphate groups attached at the 5' carbon is called a nucleotide. Apart from adenosine (A), cytidine (C), guanosine (G), thymidine (T) and uridine (U), DNA and RNA also contain bases that have been modified after the nucleic acid chain has been formed. In DNA, the most common modified base is 5-methylcytidine (m5C). In RNA, there are many modified bases, including pseudouridine (Ψ), dihydrouridine (D), inosine (I), ribothymidine (rT) and 7-methylguanosine (m7G).[1][2] Hypoxanthine and xanthine are two of the many bases created through mutagen presence, both of them through deamination (replacement of the amine-group with a carbonyl-group). Hypoxanthine is produced from adenine, xanthine from guanine.[3] Similarly, deamination of cytosine results in uracil. Structure * The "skeleton" of adenine and guanine is purine, hence the name purine-bases.
These are incorporated into the growing chain during RNA and/or DNA synthesis.
Modified purine bases These are examples of modified adenosine or guanosine.
Modified pyrimidine bases
Novel Bases A vast number of nucleobase analogues exist. The most common applications are used as fluorescent probes, either directly or indirectly, such as Aminoallyl nucleotide, which are used to label cRNA or cDNA in microarrays. Several groups are working on alternative "extra" base pairs to extend the genetic code, such as isoguanine and isocytosine or the fluorescent 2-amino-6-(2-thienyl)purine and pyrrole-2-carbaldehyde. In medicine, several nucleoside analogues are used as anticancer and antiviral agents. The viral polymerase incorporates these compounds with non-canon bases. These compounds are activated in the cells by being converted into nucleotides, they are administered as nuclosides as charged nucleotides cannot easily cross cell membranes. References 1. ^ BIOL2060: Translation
* Nucleoside
* Base pairing in DNA Double Helix (shows specific hydrogen bonds) Retrieved from "http://en.wikipedia.org/"
|