In quantum mechanics, a Slater determinant is an expression which describes the wavefunction of a multi-fermionic system that satisfies anti-symmetry (Φ = 0) requirements and subsequently the Pauli exclusion principle by changing sign upon exchange of fermions. It is named for its discoverer, John C. Slater who published Slater determinants as a means of ensuring the antisymmetry of a wave function through the use of matrices.[1][2] The Slater determinant arises from the consideration of a wave function for a collection of electrons, each with a wave function known as the spin-orbital, ![]() , where
, where ![]() denotes the position and spin of the singular electron; two electrons within the same spin orbital resulting in no wave function (Pauli's Exclusion Principle).
denotes the position and spin of the singular electron; two electrons within the same spin orbital resulting in no wave function (Pauli's Exclusion Principle).
Resolution
Two-particle case
The simplest way to approximate the wave function of a many-particle system is to take the product of properly chosen wave functions of the individual particles. For the two-particle case, we have
![]()
This expression is used in the Hartree method as an ansatz for the many-particle wave function and is known as a Hartree product. However, it is not satisfactory for fermions, such as electrons, because the wave function is not antisymmetric. An antisymmetric wave function can be mathematically described as follows:
![]()
Therefore the Hartree product does not satisfy the Pauli principle. This problem can be overcome by taking a linear combination of both Hartree products
![]()
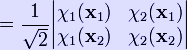
where the coefficient is the normalization factor. This wave function is antisymmetric and no longer distinguishes between fermions. Moreover, it also goes to zero if any two wave functions or two fermions are the same. This is equivalent to satisfying the Pauli exclusion principle.
Generalizations
The expression can be generalised to any number of fermions by writing it as a determinant. For an N-electron system, the Slater determinant is defined as
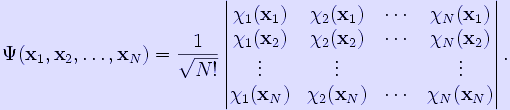
The linear combination of Hartree products for the two-particle case can clearly be seen as identical with the Slater determinant for N = 2. It can be seen that the use of (Slater) determinants assures an antisymmetrized function on the outset, symmetric functions are automatically rejected. In the same way, the use of Slater determinants assures the obeying of the Pauli principle. Indeed, the Slater determinant vanishes if the set {χi } is linearly dependent. In particular this is the case when two (or more) spinorbitals are the same. In chemistry one expresses this fact by stating that no two electrons can occupy the same spinorbital. In general the Slater determinant is evaluated by the Laplace expansion. Mathematically, a Slater determinant is an antisymmetric tensor, also known as a wedge product.
A single Slater determinant is used as an approximation to the electronic wavefunction in Hartree-Fock theory. In more accurate theories (such as configuration interaction and MCSCF), a linear combination of Slater determinants is needed.
The word "detor" was proposed by S. F. Boys to describe the Slater determinant of the general type,[3] but this term is rarely used.
See also
* Antisymmetrizer
* Electron orbital
* Quantum Electrodynamics
* Quantum Mechanics and Physical Chemistry
* Hund's Rule
* Hartree-Fock
References
1. ^ Slater, John. C. (1929). Theory of Complex Spectra Physics. Review. vol. 34 Retrieved on 13 August 2007 from "APS Physics: Physics Review Online Archive" on http://prola.aps.org/abstract/PR/v34/i10/p1293_1
2. ^ Slater, John. C. (1929)., p 1293
3. ^ Electronic wave functions I. A general method of calculation for the stationary states of any molecular system, S. F. Boys, p542, A200 (1950), Proc. Roy.Soc. (London).
Retrieved from "http://en.wikipedia.org/"
All text is available under the terms of the GNU Free Documentation License

