Moseley's law is an empirical law concerning the characteristic x-rays that are emitted by atoms. It is historically important in quantitatively justifying the conception of the nuclear model of the atom, with all or nearly all positive charges of the atom located in the nucleus, and associated on an integer basis with atomic number.
History
Using x-ray diffraction techniques in the 1910s, Henry Moseley found that the most intense short-wavelength line in the x-ray spectrum of a particular element was related to the element's atomic number, Z. This line was known as the K-alpha line. Moseley found that this relationship could be expressed by a simple formula, later called Moseley's Law.
![]()
where:
![]() is the frequency of the main or K x-ray emission line
is the frequency of the main or K x-ray emission line
![]() and
and ![]() are constants that depend on the type of line
are constants that depend on the type of line
For example, the values for ![]() and
and ![]() are the same for all Kα lines (in Siegbahn notation), so the formula can be rewritten thus:
are the same for all Kα lines (in Siegbahn notation), so the formula can be rewritten thus:
![]() z
z
Moseley himself chose to show this without k_1 \ per se, which instead was given by Moseley as a pure constant number in the standard Rydberg style, as simply 3/4 (that is, 1- 1/4) of the fundamental Rydberg frequency (3.29*1015 Hz) for K-alpha lines, and (again) for L-alpha lines according to the Rydberg formula, where k_1 \ must be 1/4 - 1/9 = 5/36 times the Rydberg frequency; this also was the way Moseley chose to write it.[1]
Moseley's ![]() was given as a general empiric constant to fit either K-alpha or L-alpha transition lines (the latter being weaker-intensity and lower frequency lines found in all X-ray element spectra, and in which case the additional numerical factor
was given as a general empiric constant to fit either K-alpha or L-alpha transition lines (the latter being weaker-intensity and lower frequency lines found in all X-ray element spectra, and in which case the additional numerical factor ![]() to modify Z is much higher). Moseley found the entire term was (Z - 7.4)2 for L-alpha transitions, and again his fit to data was good, but not as close as for K-alpha lines where the value of
to modify Z is much higher). Moseley found the entire term was (Z - 7.4)2 for L-alpha transitions, and again his fit to data was good, but not as close as for K-alpha lines where the value of ![]() was found to be 1.
was found to be 1.
Thus, Moseley's two given formulae for K-alpha and L-alpha lines, in his original semi-Rydberg style notion, (squaring both sides for clarity), are:
![]()
![]()
Derivation and justification from the Bohr model of the Rutherford nuclear atom
Moseley derived his formula empirically by plotting the square root of X-ray frequencies against a line representing atomic number. However, it was almost immediately noted (in 1914) that his formula could be explained in terms of the newly postulated 1913 Bohr model of the atom (see for details of derivation of this for hydrogen), if certain reasonable extra assumptions about atomic structure in other elements were made. However, at the time Moseley derived his laws, neither he nor Bohr could account for their form.
The 19th century empirically-derived Rydberg formula for spectroscopists is explained in the Bohr model as describing the transitions or quantum jumps between one energy level and another in a hydrogen atom. When the electron moves from one energy level to another, a photon is given off. Using the derived formula for the different 'energy' levels of hydrogen one may determine the energy or frequencies of light that a hydrogen atom can emit.
The energy of photons that a hydrogen atom can emit in the Bohr derivation of the Rydberg formula, is given by the difference of any two hydrogen energy levels:
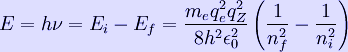
![]() = mass of an electron
= mass of an electron
![]() = charge of an electron (1.60 × 10−19 coulombs)
= charge of an electron (1.60 × 10−19 coulombs)
![]() = final energy level
= final energy level
![]() = initial energy level
= initial energy level
It is assumed that the final energy level is less than the initial energy level.
For hydrogen, the quantity ![]() because Z (the nuclear positive charge, in fundamental units of the electron charge qe) is equal to 1. That is, the hydrogen nucleus contains a single charge. However, for hydrogenic atoms (those in which the electron acts as though it circles a single structure with effective charge Z), Bohr realized from his derivation that an extra quantity Z2 would need to be added to the conventional qe4, in order to account for the extra pull on the electron, and thus the extra energy between levels, as a result of the increased nuclear charge.
because Z (the nuclear positive charge, in fundamental units of the electron charge qe) is equal to 1. That is, the hydrogen nucleus contains a single charge. However, for hydrogenic atoms (those in which the electron acts as though it circles a single structure with effective charge Z), Bohr realized from his derivation that an extra quantity Z2 would need to be added to the conventional qe4, in order to account for the extra pull on the electron, and thus the extra energy between levels, as a result of the increased nuclear charge.
In 1914 it was realized that Moseley's formula could be adapted from Bohr's, if two assumptions were made. The first was that the electron responsible for the brightest spectral line (K-alpha) which Moseley was investigating from each element, results from a transition by a single electron between the K and L shells of the atom (i.e., from the nearest to the nucleus and the one next farthest out), with energy quantum numbers corresponding to 1 and 2. Finally, the Z in Bohr's formula, though still squared, required diminishment by 1 to calculate K-alpha (after Moseley's death this would be understood as a correction to account for the screening effect on full nuclear charge Z by the remaining electron left in the K shell, eventually seen as a single 1s electron). In any case, empirically it was the quantity (Z-1) which required squaring. Thus, Bohr's formula for Moseley's K-alpha X-ray transitions became:
![]()
or (dividing both sides by h to convert E to f):
![]()
Collection of the constants in this formula into a single frequency scaling-constant gives a frequency equivalent to about 3/4 of the 13.6 eV ionization energy (see Rydberg constant for hydrogen = 3.28 x 1015 Hz), with the final value of 2.46 x 1015 Hz in good agreement with Moseley's empirically-derived value of 2.48 x 1015 Hz. This fundamental frequency is the same as that of the hydrogen Lyman-alpha line, because the 1s to 2p transition in hydrogen is responsible for both Lyman-alpha line in hydrogen, and also the K-alpha lines in X-ray spectroscopy for elements beyond hydrogen, which are described by Moseley's law. Moseley was fully aware that his fundamental frequency was Lyman-alpha, the fundamental Rydberg frequency resulting from two fundamental atomic energies, and for this reason differing by the Rydberg-Bohr factor of exactly 3/4 (see his original papers below).
However, the necessity of reduction of Z by a number close to 1 for these K-alpha lines in heavier elements (aluminum and above) was derived completely empirically by Moseley, and was not discussed by his papers in theoretical terms, since the concept of atomic shells with paired electrons was not well established in 1913 (this would not be proposed until about 1920), and in particular the Schrödinger orbitals, including the 1s orbital with only 2 electrons, would not be formally introduced and completely understoood, until 1926. At the time Moseley was puzzling over his Z-1 term with Bohr, Bohr thought that the inner shell of electrons in elements might contain at least 4 and often 6 electrons. Moseley for a time considered that this K lines resulted from a simultaneous transition of 4 electrons at once from the L to K shells of atoms, but did not commit himself on this point in this papers.
As regards Moseley's L-alpha transitions, the modern view associates electron shells with principle quantum numbers n, with each shell containing 2n² electrons, giving the n=1 "shell" of atoms 2 electrons, and the n=2 shell 8 electrons. The empirical value of 7.4 for Moseley's K2 is thus associated with n = 2 to 3, then called L-alpha transitions (not to be confused with Lyman-alpha transitions), and occurring from the "M to L" shells in Bohr's later notation. This value of 7.4 is now known to represent an electron screening effect for a fraction (specifically 7.4) of the total of 10 electrons contained in what we now know to be the n = 1 and 2 (or K and L) "shells."
Historical importance
See the biographical article on Henry Moseley for more. Moseley's formula, by Bohr's later account, not only established atomic number as a measurable experimental quantity, but gave it a physical meaning as the positive charge on the atomic nucleus (number of protons). Because of Moseley's x-ray work, elements could be ordered in the periodic system in order of atomic number rather than atomic weight. This reversed the ordering of nickel (Z=28, 58.7 u) and cobalt (Z=27, 58.9 u).
This in turn was able to produce quantitative predictions for spectral lines in keeping with the Bohr/Rutherford semi-quantum model of the atom, which assumed that all positive charge was concentrated at the center of the atom, and that all spectral lines result from changes in total energy of electrons circling it as they move from one permitted level of angular momentum and energy to another. The fact that Bohr's model of the energies in the atom could be made to calculate X-ray spectral lines from aluminum to gold in the periodic table, and that these depended reliably and quantitatively on atomic number, did a great deal for the acceptance of the Rutherford/Van den Broek/Bohr view of the structure of the atom. When later quantum theory essentially also recovered Bohr's formula for energy of spectral lines, Moseley's law became incorporated into the full quantum mechanical view of the atom, including the role of the single 1s electron which remains in the K shell of all atoms after another K electron is ejected, according to the Schroedinger equation prediction.
References
1. ^ See Moseley's 1913 paper, here transcribed by Xavier Bataille.
* Oxford Physics Teaching - History Archive, "Exhibit 12 - Moseley's graph" (Reproduction of the original Moseley diagram showing the square root frequency dependence)
Retrieved from "http://en.wikipedia.org/"
All text is available under the terms of the GNU Free Documentation License

