
Arundo donax, Photo: Michael Lahanas
Cladus: Eukaryota
Regnum: Plantae
Divisio: Magnoliophyta
Classis: Liliopsida
Subclassis: Commelinidae
Ordo: Poales
Familia: Poaceae
Subfamilia: Arundinoideae
Genus: Arundo
Species: Arundo donax
Varieties: A. donax var. versicolor
Name
Arundo donax L.
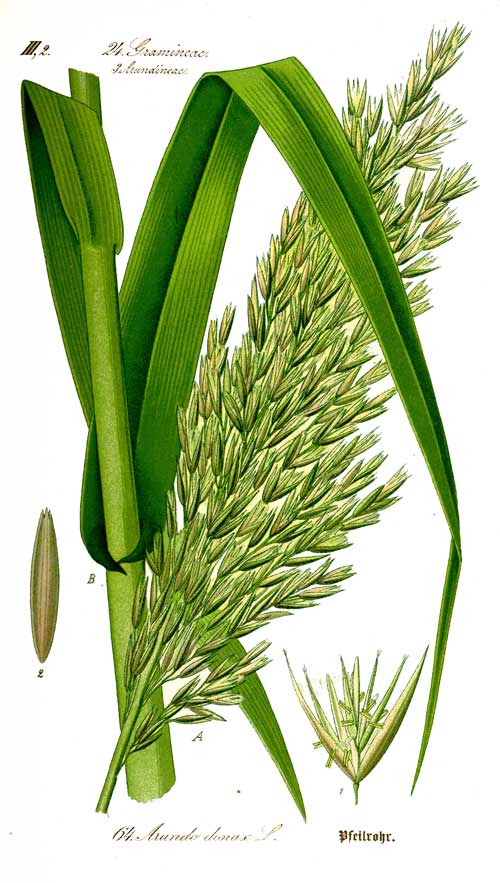
Arundo donax
Vernacular names
Internationalization
Deutsch: Pfeilrohr
Ελληνικά: Καλαμιά
English: Giant Reed
中文: 蘆荻, 芦竹
References
* USDA, NRCS. 2006. The PLANTS Database, 6 March 2006 (http://plants.usda.gov).
Data compiled from various sources by Mark W. Skinner. National Plant Data Center, Baton Rouge, LA 70874-4490 USA.
* Species Plantarum 1:81. 1753
* USDA, ARS, National Genetic Resources Program. Germplasm Resources Information Network - (GRIN) [Online Database]. [1]
-------
Arundo donax L. (Giant Cane) is a tall perennial cane growing in damp soils, either fresh or moderately saline. Other common names include Carrizo, Spanish cane, wild cane, giant reed and arundo. It is native to eastern Asia but has been widely planted and naturalised in the temperate and subtropical regions of both hemispheres (Herrera & Dudley 2003), especially in the Mediterranean, California and the Caribbean.[1][2] It forms dense stands on disturbed sites, sand dunes, in wetlands and riparian habitats.
Generally growing to 6 m, in ideal conditions it can exceed 10 m, with hollow stems 2-3 cm diameter. The leaves are alternate, 30-60 cm long and 2-6 cm broad with a tapered tip, grey-green, and have a hairy tuft at the base. Overall, it resembles an outsize common reed (Phragmites australis) or a bamboo (Subfamily Bambusoideae).
Arundo donax flowers in late summer, bearing upright, feathery plumes 40-60 cm long, but the seeds are rarely fertile. Instead, it mostly reproduces vegetatively, by underground rhizomes. The rhizomes are tough and fibrous and form knotty, spreading mats that penetrate deep into the soil up to one metre deep (Alden et al., 1998; Mackenzie, 2004). Stem and rhizome pieces less than 5 cm long and containing a single node readily sprouted under a variety of conditions (Boose and Holt, 1999). This vegetative growth appears to be well adapted to floods, which may break up individual A. donax clumps, spreading the pieces, which may sprout and colonise further downstream (Mackenzie 2004).
It uses large amounts of water from its wet habitat to supply the rapid rate of growth, up to 5 cm per day in spring (Perdue 1958). It is capable of growing in dense stands, which may crowd out other plants and prevent their growth.
Cultivation and Uses
Arundo donax has been cultivated throughout Asia, southern Europe, northern Africa, and the Middle East for thousands of years. Ancient Egyptians wrapped their dead in the leaves. The canes contain silica, perhaps the reason for their durability, and have been used to make fishing rods, walking sticks, and paper.
The stem material is both strong and flexible. It is the principal source material for reeds for woodwind instruments such as the oboe, bassoon, clarinet, and saxophone. It is also often used for the chanter and drone reeds of many different forms of bagpipes. Giant reed has been used to make flutes for over 5,000 years. The pan pipes consist of ten or more reed pipes. Its stiff stems are also used as support for climbing plants or for vines. Since Arundo species grow rapidly, their use has been suggested for biomass for energy and a source of cellulose for paper; at least one North American paper mill was considering planting it for a source of pulp fibre (Samoa Pacific, on Humboldt Bay, California, in 2002), but abandoned the plan by early 2003.
Arundo donax is strong candidate for use as a renewable biofuel source because of its fast growth rate, ability to grow in different soil types and climatic conditions. Arundo will produce an average of three kilograms of biomass per square metre (25 tons per acre) once established [3]. The energy density of the biomass produced is 17 MJ/Kg regardless of fertilizer usage [4]. Arundo donax's ability to grow for 20 to 25 years without replanting is also significant. In addition to being a biomass source, Arundo donax is also known to release volatile organic compounds (VOCs), mainly isoprene [5].
In the UK it is considered suitable for planting in and around water areas [6]
Arundo donax as an invasive species
It was introduced from the Mediterranean to California in the 1820s for roofing material and erosion control in drainage canals in the Los Angeles area (Bell 1997; Mackenzie 2004). Through spread and subsequent plantings as an ornamental plant, and for use as reeds in woodwind instruments, it has become naturalised throughout warm coastal freshwaters of North America, and its range continues to spread.
It has been planted widely through South America and Australasia (Boose and Holt 1999; Bell 1997) and in New Zealand it is listed under the National Pest Plant Accord as an "unwanted organism".[7]
It is among the fastest growing terrestrial plants in the world (nearly 10 cm/ day; Dudley, 2000). To present knowledge Arundo does not provide any food sources or nesting habitats for wildlife. This results in resources provided by the crowded-out native plants not being replaced by the Arundo (Bell 1997; Mackenzie 2004). For example, it damages California's riparian ecosystems by outcompeting native species, such as willows, for water. A. donax stems and leaves contain a variety of harmful chemicals, including silica and various alkaloids, which protect it from most insect herbivores and deter wildlife from feeding on it (Bell 1997; Miles et al. 1993; Mackenzie 2004). Grazing animals such as cattle, sheep, and goats may have some effect on it, but are unlikely to be useful in keeping it under control (Dudley 2000).
A. donax appears to be highly adapted to fires, which are unusual in native Californian riparian habitat. It is highly flammable throughout the year, and during the drier months of the year (July to October), it can increase the probability, intensity, and spread of wildfires through the riparian environment, changing the communities from flood-defined to fire-defined communities. After fires, A. donax rhizomes can resprout quickly, outgrowing native plants, which can result in large stands of A. donax along riparian corridors (Bell 1997; Scott 1994). Fire events thus push the system further toward mono-specific stands of A. donax.
A waterside plant community dominated by A. donax may also have reduced canopy shading of the in-stream habitat, which may result in increased water temperatures. This may lead to decreased oxygen concentrations and lower diversity of aquatic animals (Bell 1997).
Biological control
Biological control by specialist insect herbivores from the native range of Arundo donax is a feasible option. Although a variety of noxious chemicals are in its stems and leaves, which protect it from most insect generalist herbivores and deter wildlife from feeding on it, specialized insects are adapted to feed on Arundo and cause considerable damage (Bell, 1997; Miles et al. 1993; Mackenzie 2004, Goolsby 2007). Three of these specialist herbivore insects have been imported from Mediterranean Europe and are being evaluated as biological control agents. The three insects are the Arundo wasp, Tetramesa romana; the Arundo scale, Rhizaspidiotus donacis; and the Arundo fly, Cryptonevra spp. Other organisms that have a negative effect on Arundo donax are "Armillaria mellea (root rot), Leptostroma donacis, Papularia sphaerosperma, Puccinia coronata (crown rust), and Selenophoma donacis (stem speckle)."[8]
Mechanical control
Minor infestations can be removed manually, as long as the entire root mass and all rhizome parts are removed. Its dense growth and thick root masses make manual or mechanical removal of above-ground mass of large clonal monocultures a slow, inefficient, and difficult process. Rhizome pieces buried under 1-3 m of soil may resprout, and the disturbance caused by physical removal to the soil and surrounding communities may be severe.
Pull or dig plants, from seedlings to 2 m tall, ideally after heavy rains loosen the soil. Cut the stems of larger plants with a chainsaw or brushcutter, and dig up the roots with a shovel, pickax, or brush ax. Alternatively, use heavy equipment, such as an excavator.
Another method is to smother the plant with tarpaulin. The stems are cut as close to the ground as possible in May, and the clump covered with a very thick tarpaulin or with several tarpaulins for an entire growing season. This prevents light from reaching the plant, reducing its ability to photosynthesise, and keeps resprouts from tearing the tarpaulin. The lack of light will eventually deplete the plants' energy reserves and it will die back (Mackenzie 2004).
Chemical control
Systemic herbicides may be applied after flowering as a cut-stump treatment or foliar spray to kill the root mass (Bell 1997). Glyphosate is used in EPA-approved formulations for use in wetlands. For detailed information on the use and effects of glyphosate, see Tu et al., 2001.
Disposal
Both treated and non-treated stems can be left on-site to decompose, although they break down very slowly. If left to compost, keep the debris well away from water. For stems that have not been chemically treated and in areas where it is feasible, the debris can be burned. Otherwise, the canes can be chipped into very small pieces for mulching. Chipped material can be disposed of either in green waste containers, or spread out to dry and possibly sprayed with herbicide if any regrowth occurs from chipped debris (Mackenzie 2004).
Alkaloids
Some studies have found this plant to be rich in active tryptamine compounds, but there are more indications of the plants in India having these compounds than in the United States.[9] Toxins such as bufotenidine[10] and gramine[9] have also been found.
The dried rhizome with the stem removed has been found to contain 0.0057% DMT, 0.026% bufotenine, 0.0023% 5-MeO-MMT.[9] The flowers are also known to have DMT and the 5-methoxylated N-demethylated analogue, also 5-MeO-NMT. The quite toxic quaternary methylated salt of DMT, bufotenidine,[9] has been found in the flowers, and the cyclic dehydrobufotenidine has been found in the roots.
Some ethnobotanists and historians believe that this plant could have been used in combination with Harmal (Peganum harmala) to create a brew similar to the South American ayahuasca. This beverage is thought by some to be the mythical Soma. [11]
References
Notes
1. ^ "Catalogue of Life 2008". http://www.catalogueoflife.org/show_species_details.php?record_id=726103.
2. ^ http://ucce.ucdavis.edu/datastore/detailreport.cfm?usernumber=8&surveynumber=182 University of California website, Agriculture and Natural Resources
3. ^ Angelini, L.G., Ceccarinia, L., and Bonarib E.; European Journal of Agronomy, 22, 2005, pp 375-389
4. ^ Angelini, L.G., Ceccarinia, L., and Bonarib E.; European Journal of Agronomy, 22, 2005, pp 375-389
5. ^ Owen, S.M., Boissard, C., and Hewitt, C. N. Atmospheric Environment, 35, 2001, pp 5393–5409
6. ^ BS 7370-5 Recommendations for maintenance of water areas
7. ^ "Giant reed". Biosecurity New Zealand. http://www.biosecurity.govt.nz/pests/giant-reed. Retrieved 2009-01-13.
8. ^ However, several of these insect species have been unintentionally introduced into North America, and research is needed to determine whether additional introductions will be worthwhile. "Arundo donax". www.hort.purdue.edu. http://www.hort.purdue.edu/newcrop/duke_energy/Arundo_donax.html. Retrieved 2008-04-28.
9. ^ a b c d Erowid Arundo Donax Info Page 1
10. ^ Erowid Arundo Donax Info Page 3
11. ^ S. Ghosal, S. K. Dutta, A. K. Sanyal, and Bhattacharya, "Arundo donex L. (Graminae), Phytochemical and Pharmacological Evaluation," in the Journal of Medical Chemistry, vol. 12 (1969), p. 480.]
General References
1. Alden, P., F. Heath, A. Leventer, R. Keen, W. B. Zomfler, eds. 1998. National Audubon Society Field Guide to California. Knopf, New York.
2. Bell, G. P. 1997. Ecology and Management of Arundo donax, and approaches to riparian habitat restoration in southern California. In Plant Invasions: Studies from North America and Europe, eds. J. H. Brock, M. Wade, P. Pysêk, and D. Green. Pp. 103-113. Backhuys, Leiden, the Netherlands.
3. Boose, A. B., and J. S. Holt. 1999. Environmental effects on asexual reproduction in Arundo donax. Weeds Research 39: 117-127.
4. Dudley, T. L. 2000. Noxious wildland weeds of California: Arundo donax. In: Invasive plants of California's wildlands. C. Bossard, J. Randall, & M. Hoshovsky (eds.).
5. Herrera, A., and T. L. Dudley. 2003. Invertebrate community reduction in response to Arundo donax invasion at Sonoma Creek. Biol.Invas 5:167-177.
6. Mackenzie, A. 2004. Giant Reed. In: The Weed Workers' Handbook. C. Harrington and A. Hayes (eds.) www.cal-ipc.org/file_library/19646.pdf
7. Miles, D. H., K. Tunsuwan, V. Chittawong, U. Kokpol, M. I. Choudhary, and J. Clardy. 1993. Boll weevil antifeedants from Arundo donax. Phytochemistry 34: 1277-1279.
8. Perdue, R. E. 1958. Arundo donax – source of musical reeds and industrial cellulose. Economic Botany 12: 368-404.
9. Scott, G. 1994. Fire threat from Arundo donax. pp. 17-18 in: November 1993 Arundo donax workshop proceedings, Jackson, N.E. P. Frandsen, S. Douthit (eds.). Ontario, CA.
10. Tu, M., C. Hurd, and J. M. Randall. 2001. Weed Control Methods Handbook: Tools and Techniques for Use in Natural Areas. The Nature Conservancy.
11. Excerpted from Chapter 15 of TIHKAL, 1997
Retrieved from "http://en.wikipedia.org/"
All text is available under the terms of the GNU Free Documentation License

