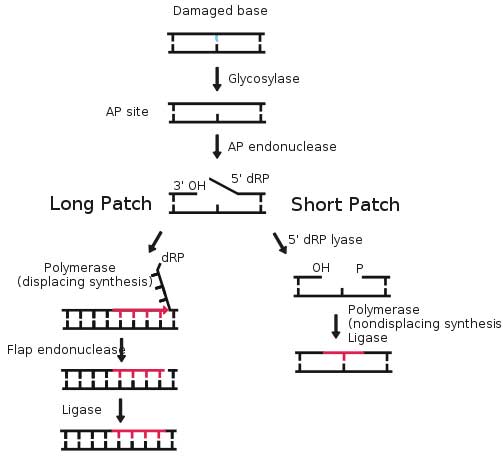
Base excision repair (BER) is a cellular mechanism that repairs damaged DNA throughout the cell cycle. It is primarily responsible for removing small, non-helix-distorting base lesions from the genome. The related nucleotide excision repair pathway repairs bulky helix-distorting lesions. BER is important for removing damaged bases that could otherwise cause mutations by mispairing or lead to breaks in DNA during replication. BER is initiated by DNA glycosylases, which recognize and remove specific damaged or inappropriate bases, forming AP sites. These are then cleaved by an AP endonuclease. The resulting single-strand break can then be processed by either short-patch (where a single nucleotide is replaced) or long-patch BER (where 2-10 new nucleotides are synthesized).[1]
Basic steps of base excision repair
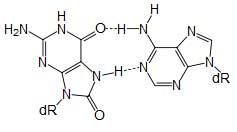
8-oxoguanine forms a Hoogsteen base pair with adenine Single bases in DNA can be chemically damaged by a variety of mechanisms, most commonly deamination, oxidation, and alkylation. These modifications can affect the ability of the base to hydrogen-bond, resulting in incorrect base-pairing, and, as a consequence, mutations in the DNA. For example, incorporation of adenine across from 8-oxoguanine (right) during DNA replication causes a G:C base pair to be mutated to T:A. Other examples of base lesions repaired by BER include: * Oxidized bases: 8-oxoguanine, 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG, FapyA) In addition to base lesions, the downstream steps of BER are also utilized to repair single-strand breaks. The choice between long patch vs. short patch repair The choice between short- and long-patch repair is currently under investigation. Various factors are thought to influence this decision, including the type of lesion, the cell cycle stage, and whether the cell is terminally differentiated or actively dividing.[3] Some lesions, such as oxidized or reduced AP sites, are resistant to pol β lyase activity and therefore must be processed by long-patch BER. Pathway preference may differ between organisms, as well. While human cells utilize both short- and long-patch BER, the yeast Saccharomyces cerevisiae was long thought to lack a short-patch pathway because it does not have homologs of several mammalian short-patch proteins, including pol β, DNA ligase III, XRCC1, and the kinase domain of PNKP. The recent discovery that the poly-A polymerase Trf4 possesses 5' dRP lyase activity has challenged this view.[4] Proteins involved in base excision repair DNA glycosylases DNA glycosylases are responsible for initial recognition of the lesion. They flip the damaged base out of the double helix, as pictured, and cleave the N-glycosidic bond of the damaged base, leaving an AP site. There are two categories of glycosylases: monofunctional and bifunctional. Monofunctional glycosylases have only glycosylase activity, whereas bifunctional glycosylases also possess AP lyase activity. Bifunctional glycosylases can therefore convert a base lesion into a single-strand break without the need for an AP endonuclease. β-Elimination of an AP site by a glycosylase-lyase yields a 3' α,β-unsaturated aldehyde adjacent to a 5' phosphate, which differs from the AP endonuclease cleavage product.[5] Some glycosylase-lyases can further perform δ-elimination, which converts the 3' aldehyde to a 3' phosphate. A wide variety of glycosylases have evolved to recognize different damaged bases. Examples of DNA glycosylases include Ogg1, which recognizes 8-oxoguanine, Mag1, which recognizes 3-methyladenine, and UNG, which removes uracil from DNA. AP endonucleases The AP endonucleases cleave an AP site to yield a 3' hydroxyl adjacent to a 5' deoxyribosephosphate (dRP). AP endonucleases are divided into two families based on their homology to the ancestral bacterial AP endonucleaes endonuclease IV and exonuclease III.[6] Many eukaryotes have members of both families, including the yeast Saccharomyces cerevisiae, in which Apn1 is the EndoIV homolog and Apn2 is related to ExoIII. In humans, only a single AP endonuclease, APE1, has been identified.[7]. It is a member of the ExoIII family. End processing enzymes In order for ligation to occur, a DNA strand break must have a hydroxyl on its 3' end and a phosphate on its 5' end. In humans, polynucleotide kinase-phosphatase (PNKP) promotes formation of these ends during BER. This protein has a kinase domain, which phosphorylates 5' hydroxyl ends, and a phosphatase domain, which removes phosphates from 3' ends. Together, these activities ready single-strand breaks with damaged termini for ligation. The AP endonucleases also participate in 3' end processing. Besides opening AP sites, they possess 3' phosphodiesterase activity and can remove a variety of 3' lesions including phosphates, phosphoglycolates and aldehydes. 3' processing must occur before DNA synthesis can initiate because DNA polymerases require a 3' hydroxyl to extend from. DNA polymerases Pol β is the main human polymerase that catalyzes short-patch BER, with pol λ able to compensate in its absence.[8] These polymerases are members of the Pol X family and typically insert only a single nucleotide. In addition to polymerase activity, these enzymes have a lyase domain that removes the 5' dRP left behind by AP endonuclease cleavage. During long-patch BER, DNA synthesis is thought to be mediated by pol δ and pol ε along with the processivity factor PCNA, the same polymerases that carry out DNA replication. These polymerases perform displacing synthesis, meaning that the downstream 5' DNA end is "displaced" to form a flap (see diagram above). Pol β can also perform long-patch displacing synthesis and can therefore participate in either BER pathway.[9] Long-patch synthesis typically inserts 2-10 new nucleotides. FEN1 removes the 5' flap generated during long patch BER. This endonuclease shows a strong preference for a long 5' flap adjacent to a 1-nt 3' flap.[10] The yeast homolog of FEN1 is RAD27. In addition to its role in long-patch BER, FEN1 cleaves flaps with a similar structure during Okazaki fragment processing, an important step in lagging strand DNA replication. DNA ligase DNA ligase III along with its cofactor XRCC1 catalyzes the nick sealing step in short-patch BER in humans. DNA ligase I ligates the break in long patch BER. Links between BER and cancer Defects in a variety of DNA repair pathways lead to cancer predisposition, and BER appears to follow this pattern. Deletion of BER genes increases the mutation rate in a variety of organisms, predicting that loss of BER could contribute to the development of cancer. Indeed, somatic mutations in Pol β have been found in 30% of human cancers, and some of these mutations lead to transformation when expressed in mouse cells.[11] Mutations in the DNA glycosylase MYH are also known to increase susceptibility to colon cancer. See also * DNA repair
1. ^ Liu Y, Prasad R, Beard WA, Kedar PS, Hou EW, Shock DD, Wilson SH, (2007). "Coordination of Steps in Single-nucleotide Base Excision Repair Mediated by Apurinic/Apyrimidinic Endonuclease 1 and DNA Polymerase beta.". The Journal of Biological Biochemistry 282 (18): 13532–13541. doi:10.1074/jbc.M611295200. PMID 17355977.
* MeSH Base+Excision+Repair Retrieved from "http://en.wikipedia.org/"
|