|
|
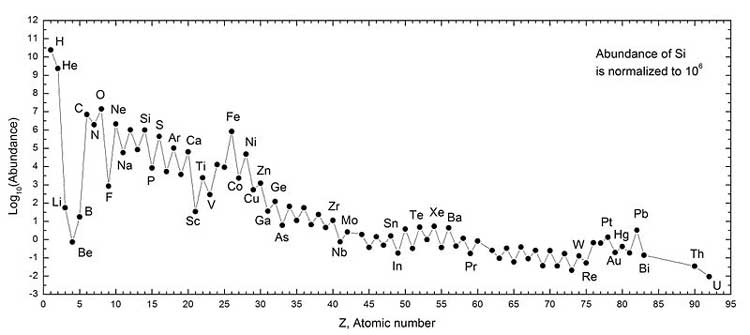
Iron peak — local maximum in the vicinity of Fe (V, Cr, Mn, Fe, Co and Ni) on the graph "abundances of the chemical elements".
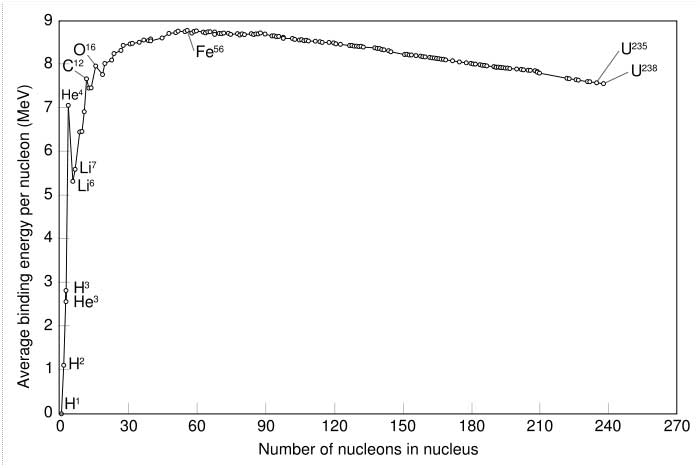
Abundances of the chemical elements: Solar system abundances (*) For elements before iron nuclear fusion releases energy. For elements heavier than iron nuclear fusion consumes energy, but nuclear fission releases it. Chemical elements up to iron peak are produced in ordinary stellar nucleosynthesis. Heavier elements are produced only during supernova nucleosynthesis. For this reason we have more iron peak elements than in its neighbourhood. Binding energy The graph below shows the binding energy of various elements. Increasing values of binding energy can be thought of in two ways: 1) it is the energy required to remove a nucleon from a nucleus, and 2) it is the energy released when a nucleon is added to a nucleus. As can be seen, light elements such as hydrogen release large amounts of energy (a big increase in binding energy) as nucleons are added—the process of fusion. Conversely, heavy elements such as uranium release energy when nucleons are removed—the process of nuclear fission. Although nuclei with 58 and 62 nucleons have the very highest binding energy, fusing four nucleons to nickel–56 to produce the next element — zinc–60 — actually requires energy rather than releases any. Accordingly, nickel–56 is the last fusion product produced in the cores of a high-mass stars (see also Silicon burning process).
(*) See also * Cosmochemical Periodic Table of the Elements in the Solar System Retrieved from "http://en.wikipedia.org/" |
|